Naisulid® (Neusulid)
Undesirable side effects can be minimized by using the drug in the minimum effective dose with the minimum duration of use necessary to relieve pain.
There is evidence of very rare cases of serious reactions from the liver, including cases of death, associated with the use of nimesulide-containing drugs. If symptoms similar to signs of liver damage appear (anorexia, itching, yellowing of the skin, nausea, vomiting, abdominal pain, dark urine, increased activity of liver transaminases), you should immediately stop using the drug Naisulide® and consult a doctor. Repeated use of Naisulide® in such patients is contraindicated.
Liver reactions, which are in most cases reversible, have been reported with short-term use of the drug.
While using the drug Naisulide®, the patient should refrain from taking other analgesics, including NSAIDs (including selective COX-2 inhibitors).
The drug Naisulide® should be used with caution in patients with a history of gastrointestinal diseases (ulcerative colitis, Crohn's disease), since exacerbation of these diseases is possible.
The risk of gastrointestinal bleeding, peptic ulcer/perforation of the stomach or duodenum increases in patients with a history of gastrointestinal ulceration (ulcerative colitis, Crohn's disease), as well as in elderly patients, with an increase in the dose of NSAIDs, so treatment should begin with the lowest possible dose. In such patients, as well as in patients who require the simultaneous use of low doses of acetylsalicylic acid or other drugs that increase the risk of complications from the gastrointestinal tract, it is recommended to additionally prescribe gastroprotectors (misoprostol or proton pump blockers). Patients with a history of gastrointestinal disease, especially older patients, should report new gastrointestinal symptoms (especially symptoms that may indicate possible gastrointestinal bleeding) to their physician.
Naisulide should be administered with caution to patients taking drugs that increase the risk of ulceration or bleeding (oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors or antiplatelet agents such as acetylsalicylic acid).
If gastrointestinal bleeding or ulcerative lesions of the gastrointestinal tract occur in patients taking Naisulide®, treatment with the drug must be discontinued.
Given reports of visual impairment in patients taking other NSAIDs, if any visual impairment occurs, use of Naisulide should be immediately discontinued and an ophthalmological examination performed.
The drug may cause fluid retention, therefore, in patients with arterial hypertension, renal and/or heart failure, Naisulide® should be used with extreme caution. If the condition worsens, treatment with Naisulide® should be discontinued.
Clinical studies and epidemiological data suggest that NSAIDs, especially at high doses and with long-term use, may lead to a small risk of myocardial infarction or stroke. There is insufficient data to exclude the risk of such events when using nimesulide.
The drug contains sucrose, this should be taken into account by patients suffering from diabetes mellitus (0.15-0.18 XE per 100 mg of the drug) and those on a low-calorie diet. The drug Naisulide® is not recommended for use in patients with fructose intolerance, sucrose-isomaltose deficiency or glucose-galactose malabsorption syndrome.
If signs of a “cold” or acute respiratory viral infection occur during the use of the drug Naisulid®, the drug should be discontinued.
Nimesulide can change the properties of platelets, so caution must be exercised when using the drug in people with hemorrhagic diathesis, however, the drug does not replace the preventive effect of acetylsalicylic acid in cardiovascular diseases.
Elderly patients are especially susceptible to adverse reactions to NSAIDs, including the risk of life-threatening gastrointestinal bleeding and perforation, and decreased renal, liver, and cardiac function. When taking the drug Naisulide® for this category of patients, proper clinical monitoring is necessary.
There is evidence of rare cases of skin reactions (such as exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis) when taking NSAIDs, including nimesulide. At the first manifestations of a skin rash, damage to the mucous membranes or other signs of an allergic reaction, taking Naisulide® should be stopped immediately.
Naisulide powder for the preparation of suspension for oral administration 100 mg in 2.0 g bags No. 10
Name
Naisulide por.drip.susp.dn.approx.100mg in 2.0g pack in pack No. 10
Description
Powder of white or white with a yellowish tint color with a specific odor. By adding 100 ml of carbon dioxide-free water (freshly boiled and cooled water) to the contents of the bag, a white or light yellow suspension with a characteristic orange odor is obtained.
Main active ingredient
Nimesulide
Release form
powder
Dosage
100mg
Special instructions and precautions
Adverse effects can be minimized by using the lowest effective dose for the minimum amount of time needed to relieve symptoms. If the patient's condition does not improve, treatment should be stopped. Elderly Patients Elderly patients have an increased incidence of adverse reactions with NSAIDs, especially gastrointestinal bleeding and perforation (in some cases fatal), and an increased incidence of renal, hepatic and cardiac dysfunction. Therefore, appropriate close clinical monitoring is recommended. Concomitant therapy When using the drug Naisulide®, the simultaneous use of other NSAIDs, including selective cyclooxygenase-2 inhibitors, should be avoided. You should refrain from taking other analgesics at the same time (see also section “Interaction with other medicinal products and other forms of interaction”). Effect on the liver In rare cases, serious reactions from the liver may occur associated with the use of nimesulide-containing drugs, including in very rare cases death. If patients taking nimesulide develop symptoms or signs that may indicate liver damage and impaired liver function (for example, anorexia, nausea, vomiting, abdominal pain, fatigue, dark urine, abnormal laboratory parameters characterizing liver function ), treatment with nimesulide should be discontinued. Such patients are not recommended to take nimesulide in the future. With short-term use of the drug Naisulide®, liver damage is usually reversible. Patients who develop fever and/or other flu-like or cold-like symptoms while using nimesulide should immediately stop using nimesulide. Gastrointestinal disorders Gastrointestinal bleeding, ulceration or perforation of ulcers may occur during the use of any NSAID at various stages of treatment, regardless of the presence of warning symptoms or a history of gastrointestinal pathology. Patients with any history of gastrointestinal disorders, especially elderly patients, should report any symptoms occurring in the gastrointestinal tract. This is especially important in the initial stages of treatment. If ulcers, bleeding or other complications develop, nimesulide should be discontinued. Gastrointestinal bleeding, ulcers and ulcer perforation that occur during the use of nimesulide may threaten the patient's life, especially if there is a history (regardless of the elapsed time) of the occurrence of such conditions during treatment with any NSAIDs with or without the presence of dangerous symptoms or history contains indications of other serious disorders of the gastrointestinal tract. The risk of gastrointestinal bleeding, ulcers, and perforation of ulcers is increased when taking high doses of nimesulide, as well as in patients with a history of ulcers, especially with ulcers complicated by bleeding or perforation, in the elderly. These patients should start treatment with the lowest dose. For these patients, as well as patients who are concomitantly taking low-dose aspirin or other drugs that increase the risk of gastrointestinal disease, consideration should be given to adding an agent to the treatment regimen that protects the gastrointestinal mucosa from damage (eg, misoprostol). or proton pump inhibitor). Naisulide should be used with caution in patients with gastrointestinal pathologies, including peptic ulcers, a history of gastrointestinal bleeding, ulcerative colitis and Crohn's disease, due to the risk of exacerbation of these conditions. It should also be used with caution in patients taking other medications that may increase the risk of ulceration or bleeding (eg, oral corticosteroids, anticoagulants (warfarin), selective serotonin reuptake inhibitors, antiplatelet agents (aspirin); see also section "Interactions with other drugs" means and other forms of interaction"). Skin reactions There is evidence of very rare cases of severe skin reactions with the use of NSAIDs (including exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis); such reactions can lead to death. Patients appear to be at greatest risk of developing skin reactions during the initial period of therapy. Naisulide should be discontinued at the first sign of skin rash, damage to the mucous membranes and/or other hypersensitivity reactions. Impaired renal function In patients with renal or heart failure, nimesulide should be used with caution as nimesulide may worsen renal function. If the condition worsens, treatment should be discontinued. Cardiovascular and cerebrovascular effects It is necessary to monitor the condition of patients with arterial hypertension and/or a history of mild to moderate heart failure, since cases of fluid retention and the development of edema have been reported during treatment with NSAIDs. Clinical studies and epidemiological data suggest that some NSAIDs, especially in high doses and with long-term use, may lead to a slight increase in the risk of pathological conditions associated with arterial thrombosis (for example, myocardial infarction or stroke). There is insufficient data to exclude the risk of such conditions when using nimesulide. In patients with uncontrolled hypertension, congestive heart failure, established coronary artery disease, peripheral arterial disease and/or cerebrovascular disease, nimesulide should be prescribed after a careful assessment of the condition and benefit/risk ratio. Also, a thorough assessment of the condition and benefit/risk ratio should be performed before starting long-term treatment in patients with risk factors for cardiovascular disease (for example, hypertension, hyperlipidemia, diabetes mellitus, smoking). Since nimesulide can affect platelet function, it should be prescribed with caution to patients with bleeding diathesis. Naisulide® cannot serve as a replacement for acetylsalicylic acid in the prevention of cardiovascular diseases. Effect on fertility The use of nimesulide can reduce female fertility, so it is not recommended for women planning pregnancy. In women who have problems conceiving or who are being evaluated for infertility, discontinuation of nimesulide should be considered. Excipients Naisulide® contains sucrose (white crystalline sugar). This medicine should not be prescribed to patients with rare hereditary disorders such as fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase deficiency. Due to the sucrose content, the drug may have a negative effect on tooth enamel.
Pharmacological properties
Nimesulide is a non-steroidal anti-inflammatory drug with analgesic and antipyretic properties that acts by inhibiting the enzyme cyclooxygenase involved in the synthesis of prostaglandins. When taken orally, it is well absorbed. Nimesulide is actively metabolized in the liver in various ways, including with the help of cytochrome P450 CYP2C9, the activity of which decreases, which can lead to an increase in the blood plasma concentration of drugs taken simultaneously with nimesulide and which, like nimesulide, are a substrate of this isoenzyme. The main metabolite is the para-hydroxy form, which has pharmacological activity. Hydroxynimesulide is the only metabolite that can be detected in plasma and is almost entirely in the conjugated state. Nimesulide is excreted mainly in the urine (approximately 50% of the administered dose). Only 1–3% is excreted unchanged. Approximately 29% of the administered substance is excreted after biotransformation in the feces. The kinetic profile of nimesulide does not change in elderly patients with single and repeated administration. In an experimental study with mild to moderate renal dysfunction (creatinine clearance 30 × 80 ml/min), the Cmax of nimesulide and its metabolites did not exceed the level in healthy volunteers. AUC and T1/2 were 50% higher, but always within the values observed when using nimesulide in healthy volunteers. Repeated use of the drug does not lead to accumulation. Contraindicated in patients with liver failure.
Indications for use
- Treatment of acute pain.
- Primary dysmenorrhea.
Naisulide® can only be prescribed as a second-line treatment. The decision to prescribe nimesulide should be based on an overall risk assessment for each patient.
Directions for use and doses
The medicine is intended for oral administration. Before use, it is necessary to prepare a suspension. To do this, the contents of the package are dissolved in 100 ml of freshly boiled drinking water cooled to room temperature and shaken vigorously. After this, the suspension is ready for use; it must be taken immediately after preparation. Undesirable effects of therapy can be minimized by administering the lowest effective dose of the drug for the shortest possible period of time required to treat the disease in question. The maximum duration of taking nimesulide should not exceed 15 days. Adult patients: 1 sachet (100 mg of nimesulide) 2 times a day after meals. Elderly patients: When treating elderly patients, there is no need to reduce the daily dose. Children and adolescents Children (under 12 years of age): for this category of patients, the administration of nimesulide-containing medications is contraindicated (see section “Contraindications”). Adolescents (12 to 18 years): Given the pharmacokinetic profile of nimesulide in adults and the pharmacodynamic characteristics of nimesulide, there is no need for dose adjustment in adolescents. Patients with impaired renal function: given the pharmacokinetic data, there is no need for dose adjustment in patients with mild to moderate renal impairment (creatinine clearance 30 × 80 ml/min); in severe renal failure (creatinine clearance
Use during pregnancy and lactation
Fertility Like other NSAIDs, nimesulide is not recommended for women planning pregnancy (see also section "Special instructions and precautions"). Pregnancy Inhibition of prostaglandin synthesis can negatively affect the course of pregnancy, the development of the embryo/fetus, and the course of labor. Nimesulide, which inhibits prostaglandin synthetase, can lead in the fetus to premature closure of the ductus arteriosus, pulmonary hypertension, renal dysfunction (which can progress to renal failure with oligohydramnios), and can also prolong bleeding time and inhibit uterine contractions. Data from epidemiological studies show an increased risk of miscarriage and heart defects following the use of prostaglandin synthesis inhibitors early in pregnancy. The risk is expected to increase with dose and duration of treatment. In animals, the use of prostaglandin synthesis inhibitors led to an increase in pre- and postimplantation losses and fetal/embryo mortality. In addition, in animals after administration of prostaglandin synthesis inhibitors during organogenesis, an increased incidence of various malformations, including cardiovascular diseases, was observed. Tests in rabbits revealed atypical reproductive toxicity. There are no data on the use of nimesulide in pregnant women. Therefore, the potential risk to humans cannot be reliably assessed and the use of nimesulide during pregnancy is contraindicated. Lactation It is not known whether nimesulide is excreted into breast milk. Therefore, its use is contraindicated during breastfeeding.
Interaction with other drugs
Pharmacodynamic interactions Other non-steroidal anti-inflammatory drugs (NSAIDs) The combined use of drugs containing nimesulide and other non-steroidal anti-inflammatory drugs, including acetylsalicylic acid in anti-inflammatory doses (? 1 g once or ? 3 g as a total daily dose), is not recommended. Corticosteroids Corticosteroids increase the risk of gastrointestinal ulcers or bleeding. Anticoagulants NSAIDs may enhance the effects of anticoagulant agents such as warfarin. Due to the increased risk of bleeding, this combination is not recommended for patients receiving warfarin, acetylsalicylic acid, or other drugs with anticoagulant effects. This combination is contraindicated in patients with severe bleeding disorders. If combination therapy cannot be avoided, careful monitoring of blood clotting parameters is necessary. Antiplatelet agents and selective serotonin reuptake inhibitors Antiplatelet agents and selective serotonin reuptake inhibitors increase the risk of gastrointestinal bleeding. Diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, and NSAIDs may reduce the effectiveness of diuretics and other antihypertensive drugs. In some patients with impaired renal function (for example, in dehydrated or elderly patients), co-administration of ACE inhibitors or angiotensin II receptor antagonists, as well as substances that inhibit the cyclooxygenase system, may cause a further decrease in renal function (up to acute renal failure). ), which is usually reversible. This interaction should be taken into account in patients taking Naisulide together with ACE inhibitors or angiotensin II receptor antagonists. Caution should be exercised when prescribing this combination, especially in elderly patients. Patients should be kept adequately hydrated and the need to monitor renal function should be considered when initiating combination therapy and periodically thereafter. Pharmacokinetic interactions: effects of nimesulide on the pharmacokinetics of other drugs Furosemide In healthy volunteers, nimesulide temporarily reduced the effect of furosemide on sodium excretion, to a lesser extent on potassium excretion, and also reduced the diuretic effect. Co-administration of nimesulide and furosemide leads to a decrease (by approximately 20%) in the area under the concentration-time curve (AUC) and a decrease in the cumulative excretion of furosemide without changing the renal clearance of furosemide. Co-administration of furosemide and drugs containing nimesulide requires caution in patients with impaired renal and/or cardiac function. Lithium There is evidence that NSAIDs reduce the clearance of lithium, resulting in increased plasma lithium levels and thus an increased likelihood of lithium toxicity. When prescribing the drug Naisulide® to patients receiving lithium-based therapy, frequent monitoring of lithium levels in the blood plasma should be carried out. Methotrexate When prescribing nimesulide less than 24 hours before or less than 24 hours after taking methotrexate, caution is required, since in such cases the level of methotrexate in plasma and, accordingly, the toxic effects of this drug may increase. Cyclosporine Due to their effect on renal prostaglandins, inhibitors of prostaglandin synthetase, which include nimesulide, may increase the nephrotoxicity of cyclosporine. Other agents In vivo studies have been conducted to identify possible pharmacokinetic interactions with glibenclamide, theophylline, warfarin, digoxin, cimetidine and antacids (for example, a combination of aluminum hydroxide and magnesium hydroxide). No clinically significant interactions were observed. Nimesulide inhibits the activity of the CYP2C9 enzyme. With the simultaneous use of nimesulide and drugs that are substrates of this enzyme, the concentration of these drugs in the blood plasma may increase. Pharmacokinetic interactions: effects of other agents on the pharmacokinetics of nimesulide In vitro studies have shown that nimesulide is displaced from binding sites by tolbutamide, salicylic acid and valproic acid. Other than a possible effect on plasma levels, this interaction is not expected to have any clinical significance.
Contraindications
- Known individual hypersensitivity to nimesulide and/or other components of the drug.
- In the anamnesis? hypersensitivity (for example, bronchospasm, rhinitis, urticaria, etc.) to acetylsalicylic acid and other non-steroidal anti-inflammatory drugs.
- In the anamnesis? hepatotoxic reactions to nimesulide.
- Concomitant use with other potentially hepatotoxic drugs.
- Liver failure.
- Severe renal impairment (creatinine clearance
- Gastric or duodenal ulcer in the acute phase, a history of recurrent ulcers or bleeding in the gastrointestinal tract, cerebral hemorrhages or other disorders accompanied by bleeding.
- Severe blood clotting disorders.
- Severe heart failure.
- Pregnancy and breastfeeding period.
- Children's age up to 12 years.
- Alcoholism, drug addiction.
- Fever and/or flu-like symptoms.
Compound
2.0 g of powder contains 100 mg of nimesulide as an active ingredient. Excipients: maltodextrin, anhydrous citric acid, Orange PX 1488 flavor, white crystalline sugar.
Overdose
An acute overdose of nimesulide can be manifested by apathy, drowsiness, nausea and vomiting, pain in the epigastric region, which decrease with symptomatic treatment. Overdose can lead to gastrointestinal bleeding, rarely? to hypertension, acute renal failure, decreased respiratory activity and coma. In case of nimesulide overdose, symptomatic and supportive therapy is indicated. There is no specific antidote for nimesulide. There is no information on the elimination of nimesulide during hemodialysis, but since nimesulide is characterized by a high degree of binding to plasma proteins (97.5%), hemodialysis is most likely useless in the treatment of overdose. Due to the strong binding to plasma proteins, neither forced diuresis, urine alkalinization, nor hemoperfusion are likely to be effective. If an overdose has occurred within the last 4 hours or an overdose of very high doses is observed, then induce vomiting and/or take activated charcoal (60 x 100 g for adults) and/or an osmotic laxative. In case of overdose, renal and liver functions should be carefully monitored.
Side effect
Clinical studies and epidemiological data suggest that the use of some NSAIDs, especially in high doses and for long periods of time, may be associated with a slight increase in the risk of arterial thrombosis (eg, myocardial infarction or stroke). Edema, increased blood pressure, and heart failure have also been reported during treatment with NSAIDs. There is evidence of very rare cases of severe skin reactions (including Steven-Jones syndrome and toxic epidermal necrolysis) with the use of NSAIDs. When treated with NSAIDs, the most common adverse events were gastrointestinal events. Peptic ulcers, perforation or gastrointestinal bleeding may develop, sometimes with fatal consequences, especially in elderly patients. There is information about the appearance of nausea, vomiting, diarrhea, flatulence, constipation, dyspepsia, abdominal pain, tarry stools, vomiting blood, ulcerative stomatitis, exacerbation of colitis and Crohn's disease after taking the drug. Gastritis is less common. The following list of adverse reactions is based on the results of controlled clinical trials* (involving approximately 7800 people) and post-marketing experience. Reactions are distributed by type and frequency of occurrence. The frequency of occurrence of possible adverse reactions is indicated as: very often (? 1/10); often (from ? 1/100 to
- Known individual hypersensitivity to nimesulide and/or other components of the drug.
- In the anamnesis? hypersensitivity (for example, bronchospasm, rhinitis, urticaria, etc.) to acetylsalicylic acid and other non-steroidal anti-inflammatory drugs.
- In the anamnesis? hepatotoxic reactions to nimesulide.
- Concomitant use with other potentially hepatotoxic drugs.
- Liver failure.
- Severe renal impairment (creatinine clearance
- Gastric or duodenal ulcer in the acute phase, a history of recurrent ulcers or bleeding in the gastrointestinal tract, cerebral hemorrhages or other disorders accompanied by bleeding.
- Severe blood clotting disorders.
- Severe heart failure.
- Pregnancy and breastfeeding period.
- Children's age up to 12 years.
- Alcoholism, drug addiction.
- Fever and/or flu-like symptoms.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25 °C. Keep out of the reach of children.
NIMESULIDE
special instructions
Since iimesulide is partially eliminated by the kidneys, its dose in patients with impaired renal function should be reduced depending on creatinine clearance.
Considering reports of visual impairment in patients taking other NSAIDs. Treatment should be stopped immediately if any visual disturbance occurs and the patient should be examined by an ophthalmologist.
The drug can cause fluid retention in tissues, so patients with high blood pressure and cardiac disorders should use nimesulide with extreme caution.
Patients should undergo regular medical monitoring if they, along with nimesulide, take medications that are known to affect the gastrointestinal tract. If signs of liver damage appear (itching, yellowing of the skin, nausea, vomiting, abdominal pain, dark urine, increased activity of liver transaminases), you should stop taking the drug and consult your doctor. The drug should not be used simultaneously with other NSAIDs.
During treatment with nimesulide, it is recommended to avoid the simultaneous use of hepatotoxic drugs, analgesics, other NSAIDs (with the exception of low doses of acetylsalicylic acid used in antiplatelet doses) and the use of ethanol does not replace the preventive effect of acetylsalicylic acid in cardiovascular diseases and diseases.
The use of the drug may adversely affect female fertility and is not recommended for women planning pregnancy.
After 2 weeks of using the drug, monitoring of biochemical indicators of liver function is necessary.
Gastrointestinal bleeding or ulcer/perforation may develop at any time while using the drug, with or without clinically significant symptoms. both with and without a history of gastrointestinal complications. The risk of their development is higher when high doses of NSAIDs are prescribed, when taken by patients with a history of gastric and duodenal ulcers, as well as by the elderly. If therapy is necessary in these cases, the need for concomitant use of misoprostol or proton pump inhibitors should be considered. If gastrointestinal bleeding or gastrointestinal ulcers occur, the drug should be discontinued.
Elderly patients most often develop side effects when taking the drug, including gastrointestinal bleeding, perforation, dysfunction of the heart, kidneys, and liver, so regular clinical monitoring of the condition of these patients is recommended.
Naisulide gel for 30g No. 1
Name
Naisulide gel dnar.approx. 10 mg 1 g in tubes 30 g in pack No. 1
Description
Homogeneous transparent or almost transparent yellow gel. Air bubbles are allowed.
Main active ingredient
Nimesulide
Release form
gel
Dosage
30g
special instructions
You should consult your doctor before using the gel. Naisulide® gel should be used with caution when:
- peptic ulcer in the acute phase or severe bleeding disorder;
- renal dysfunction;
- impaired liver function, with moderate and severe liver failure;
- bronchial asthma, allergic rhinitis;
- prescribed to elderly patients with impaired renal function, liver function, and congestive heart failure.
Naisulide® Gel should not be used concomitantly with other topical medications. Do not apply the drug to skin affected by dermatoses or infectious processes, to damaged areas of the skin, or to open wounds. It is necessary to avoid getting the gel into the eyes and mucous membranes. The gel should not be used under airtight dressings. If local skin reactions occur, the gel must be discontinued. By using a low effective dose for short-term use, side effects can be reduced. In exceptional cases, burning sensations may occur on the skin: photo dermatitis; Caution must be exercised during treatment. Naisulide® gel. To reduce the risk of photosensitivity, patients should avoid exposure to direct sunlight or tanning lamps. If symptoms persist or the condition worsens, you should consult a doctor.
Indications for use
acute painful manifestations that occur with uncomplicated injuries, including sports ones; for sprains or ruptures of ligaments and tendons, tendinitis; bruises of muscles and ligaments.
Directions for use and doses
For external use, wash and dry the skin surface before applying the gel. Approximately 3 cm of gel is applied to the affected area and rubbed in lightly; the procedure is repeated 3? 4 times a day. Do not rub the gel vigorously or use bandages. After applying the gel, you must wash your hands thoroughly, except in cases where the drug must be applied directly to your hands. The duration of treatment is selected individually; is usually 7? 15 days. Long-term use of the drug is carried out under mandatory systematic monitoring of the patient’s kidney and liver function.
Use during pregnancy and lactation
Naisulid® gel is contraindicated for use during pregnancy and lactation. If it is necessary to prescribe the drug during lactation, it is necessary to decide on stopping breastfeeding.
Interaction with other drugs
When using the drug cutaneously, its interaction with other drugs has not been established. By displacing some drugs from the sites where they bind to plasma proteins, nimesulide can significantly increase both their effectiveness and the toxicity of these drugs. The drug should be prescribed with caution simultaneously with anticoagulants, digoxin, phenytoin, lithium drugs, diuretics, antihypertensive drugs, other NSAIDs, cyclosporine, methotrexate, and oral hypoglycemic drugs. With simultaneous local use of several NSAIDs, skin redness, peeling, and urticaria may occur.
Contraindications
- hypersensitivity to nimesulide or other components of the drug;
- increased sensitivity to non-steroidal anti-inflammatory drugs;
- dermatitis and infectious skin diseases;
- damage to the epidermis;
- renal failure (creatinine clearance less than 30 ml/min);
- pregnancy and lactation;
- children's age up to 12 years.
Compound
1 g of gel contains 10 mg of nimesulide as an active ingredient. Excipients: ethyl alcohol 96%, carbomer, macrogol 400, propylene glycol, dimethyl sulfoxide.
Overdose
There were no cases of overdose when using the gel. When using the gel on large areas of the skin or if recommended doses are exceeded, systemic side effects characteristic of nimesulide and other NSAIDs are possible: dyspepsia, headache, dizziness, pain in the epigastric region. Treatment: symptomatic. A dose reduction or discontinuation of the drug is required.
Side effect
The incidence of side effects is assessed according to the following scheme: very often (? 1/10); often (? 1/100 to ? 1/10); infrequently (? 1/1000 to ? 1/100); rare (? 1/10000 to ? 1/1000); very rare (? 1/10000). On the part of the skin and subcutaneous tissue: in isolated cases, erythema, local irritation, rash, itching, peeling, change in skin color (especially with prolonged use of the gel) in the form of skin hyperemia and allergic reactions are possible. From the respiratory system, chest and mediastinal organs: rarely? Quincke's edema, vasomotor rhinitis, suffocation, bronchospasm. If the listed adverse reactions occur, as well as a reaction not listed in the package insert, you should stop using the gel and consult a doctor.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25 °C. Keep out of the reach of children.
Long-term use of nimesulide in real clinical practice: safety issues
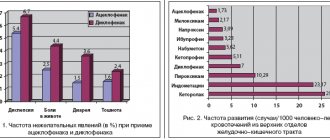
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used drugs in real clinical practice. The combination of analgesic, anti-inflammatory and antipyretic effects has led to the demand for these drugs in the treatment of a large number of diseases. At the same time, NSAIDs occupy a different place in the treatment of different nosological forms. For a number of diseases, NSAIDs are primarily symptomatic agents (for example, for cancer), although in fairness it should be noted that a number of NSAIDs (for example, nimesulide and celecoxib) have the ability to slow down neoangiogenesis and inhibit tumor growth [1]. For acute pain in the spine, anti-inflammatory drugs, along with analgesics, are used in short courses until the pain syndrome and local inflammation decrease. A number of studies have shown that simple analgesics (paracetamol) have a comparable or lesser analgesic effect than NSAIDs in this category of patients [2]. There are diseases for which NSAIDs are considered disease-modifying (disease-modifying) drugs. Such diseases include spondyloarthritis (SpA). SpA is a group of systemic inflammatory diseases, which are characterized by frequent involvement of the sacroiliac joints and spine in the inflammatory process, the presence of peripheral mono- or oligoarthritis, seronegativity for rheumatoid factor, absence of rheumatoid nodules, family cases of the disease, association with the HLA-B27 antigen, frequent development of enthesitis, typical extra-articular manifestations (uveitis, damage to the skin and mucous membranes, inflammatory bowel diseases, etc.) [3]. According to some studies, NSAIDs are drugs, the use of which inhibits the progression of the formation of syndesmophytes in patients with SpA, and this positive effect is directly related to the NSAID consumption index, which reflects the frequency and duration of taking a particular drug in this group [4]. NSAIDs, according to the recommendations of the International Society for the Assessment of Spondyloarthritis - ASAS/EULAR (the Assessment of Spondyloarthritis International Society/European Lique Against Rheumatism), are first-line drugs in the treatment of axial SpA in the presence of pain and stiffness. Moreover, long-term use of NSAIDs is indicated for patients with persistent activity and persistence of symptoms of the disease. When prescribing NSAIDs, the risk of cardiovascular, gastrointestinal and renal pathologies should be taken into account [5]. Adverse events associated with taking NSAIDs, of which the most significant are ulcerogenic, hepato- and nephrotoxic effects, and the adverse effects of drugs on the cardiovascular system, significantly limit their long-term use [6, 7]. The recommendations of a number of rheumatological societies indicate the use of NSAIDs in minimally effective doses for a short time or recommend their replacement with analgesics [7]. Thus, when it comes to the treatment of patients with SpA, there is some contradiction in the existing recommendations: on the one hand, NSAIDs are disease-modifying drugs for this group of patients and should be used long-term, on the other hand, the possible side effects of NSAIDs make it necessary to take these drugs minimal doses and courses. What are the requirements for an optimal anti-inflammatory drug for the treatment of SpA? Firstly, a rapid anti-inflammatory and analgesic effect that lasts for a long time is necessary. Secondly, the drug must be administered orally and be highly safe. In the population of patients with SpA, the risk of rapid exacerbation of cardiovascular diseases (in particular, heart failure) is less than in other joint diseases (patients are mainly younger than 50 years). At the same time, with long-term use of NSAIDs, this risk may increase [8], which will require a search for the safest drug for therapy in the presence of cardiovascular pathology, as well as monitoring the state of the cardiovascular system. With long-term use of NSAIDs, the risk of gastrointestinal complications increases. It can be reduced by using drugs that are selective or partially selective for cyclooxygenase-2 (COX-2). If the risk of complications is high, you should take a combination of such drugs with a proton pump inhibitor. Prevention of hepato- and nephrotoxic effects with long-term use of NSAIDs seems to be the most difficult task, because these side effects are difficult to predict and may occur in individuals with no history of risk factors [9–13]. In modern clinical practice, one of the most attractive NSAIDs for chronic use is nimesulide (Nise®), an oral drug with a partially selective effect on cyclooxygenase-2 (COX-2), a low risk of complications from the cardiovascular system and minimal hepatotoxicity. - and nephrotoxic effects. Over 28 years of use (since 1985), nimesulide has been studied quite well and has found widespread use in global clinical practice [14]. The drug is used in almost 50 countries around the world. According to the pharmaceutical company Helsinn Healthcare SA, by 2005, about 450 million therapeutic courses of nimesulide were administered worldwide [15–17]. The group studying the effectiveness of nimesulide (Consensus Report Group on Nimesulid, 2006) identified the main advantages of the drug, allowing its use in clinical practice: a high safety profile regarding complications from the gastrointestinal tract (GIT), high cardiovascular safety, pronounced anti-inflammatory and analgesic effect with a rapid onset of action (30 minutes after administration) [15]. These properties of the drug are based not only on the primary suppression of COX-2 activity, but also on effects not associated with suppression of COX activity. These effects lie in the ability of the drug to control pro-inflammatory mediators, the synthesis of which occurs under the influence of 3,5-adenosine monophosphate; in the ability to influence the degranulation of mast cells, reducing the release of histamine and hydrochloric acid from the gastric mucosa. They are also due to the biochemical properties of the drug - the high lipophilicity and alkaline properties of the nimesulide molecule make it difficult to penetrate into the mucous membrane of the upper gastrointestinal tract, excluding contact irritation [15, 16, 18–21]. The maximum daily dose of Nise® is 200 mg. However, it is known that some doctors and patients refuse to use nimesulide due to reports of its negative effect on liver and kidney function [9, 22–24]. In total, about 200 cases of hepatotoxic effects of nimesulide have been described, of which 81 cases were severe. It follows from this that acute liver failure when taking nimesulide is an unpredictable effect of the drug and can develop both with its long-term use and after several doses. However, the overall incidence of adverse events from the liver is low. According to Traversa G. et al. (2003), the frequency of changes in liver function in patients taking NSAIDs in the period from 1997 to 2001 was 29.8 per 100 thousand patient-years (n = 400,000). The relative risk of developing liver pathology when using nimesulide was 1.4 (95% CI; 1.0–2.1) compared with that in patients who did not take NSAIDs for more than 12 months. The incidence of liver damage reached 35.3 per 100 thousand patient-years during treatment with nimesulide, 39.2 with diclofenac, 66.8 with ketorolac and 44.6 with ibuprofen [25]. Thus, the described 200 cases of acute liver damage in 450 million courses of drug use should be considered as a rare effect, which (according to the conclusions of a number of societies for the study of drug safety) cannot be a basis for prohibiting the use of nimesulide in clinical practice. When nimesulide is used, the literature describes a decrease in glomerular filtration rate (GFR) and the development of tubulointerstitial nephritis [9–13]. In the presence of isolated clinical cases of acute kidney injury [9, 23], the overall incidence of renal dysfunction during long-term treatment with nimesulide is unknown. The most dangerous adverse event that determines the mortality of patients using NSAIDs remains gastrointestinal bleeding. Despite preventive measures taken, thousands of cases of new gastrointestinal bleeding associated with NSAID use are reported every year [26]. According to Laporte J. et al. (2004), 401.4 cases of bleeding in the upper gastrointestinal tract were registered per 1 million inhabitants over the age of 18 years. The risk rate of gastrointestinal bleeding with nimesulide was 3.2 (95% CI: 1.9, 5.6), and with meloxicam - 5.7 (95% CI: 2.2, 15.0) [27 ]. A study was carried out at the Institute of Rheumatology of the Russian Academy of Medical Sciences (Moscow) in which 200 mg of nimesulide or 100 mg of diclofenac sodium were prescribed to patients immediately after treatment for gastric ulcer or erosive gastritis. Recurrence of ulcer after 2 months. observations occurred in only 1 patient receiving nimesulide (5.6%) and in 1/3 (33.3%) of patients treated with diclofenac (p <0.05) [17, 28, 29]. It should be noted that due to the rarity of hepato- and nephrotoxic effects and the high safety profile with regard to gastrointestinal and cardiovascular complications, the benefit/risk ratio of nimesulide remains consistently high, despite the reported cases of acute kidney and liver damage. These data were the basis for the conclusion of the Consensus Report Group on Nimesulid (2006), which did not recommend removing the drug from clinical practice. At the same time, it is known that over-the-counter dispensing of nimesulide in the Russian Federation leads to the fact that some patients take the drug in violation of the recommended dosage regimen. Therefore, it seemed interesting to us to analyze the characteristics of taking nimesulide in patients with SpA in real clinical practice with the study of patients’ awareness of the side effects of the drug and safety issues of its use, incl. and in case of violation of the recommended schemes. The purpose of this study was to study the incidence of liver and/or renal dysfunction in patients with axial SpA taking nimesulide (Nise®), and their association with the characteristics of the drug in real clinical practice. Materials and methods The study included 96 patients (18–50 years old) who were treated in the rheumatology department of the regional clinical hospital in Saratov in 2010–2013. The diagnosis of axial SpA was established based on the patient’s compliance with the ASAS (the Assessment of Spondyloarthritis International Society) classification criteria [30, 31]. Patients underwent a general clinical examination, a complete blood count (CBC), a complete urinalysis (UCA), and CRP were examined using a highly sensitive method using Diasis reagents; total protein, albumin, urea, creatinine, glucose, bilirubin, serum aspartate and alanine aminotransferase. In some patients, daily proteinuria was examined, renal ultrasound, and duplex examination of the renal arteries were performed. The presence of hyposthenuria was determined when the specific gravity of morning urine decreased to less than 1018 c.u. against the background of dry eating. GFR was calculated using the MDRD (Modification of Renal Disease Study) formula [32]. SpA activity was determined by calculating the BASDAI index (Bath Ankylosing Spondylitis Disease Activity Index) [29]. The therapy that patients received on an outpatient basis until hospitalization and the recommendations of their attending physicians upon discharge were analyzed. The presence of chronic kidney disease (CKD) was determined based on the recommendations of K/DOQI (2002) [33]. Patients taking nimesulide (Nise®) were provided with a questionnaire specifying the duration, frequency of administration and dosages of the drug used. The study protocol was approved by the ethical committee of the State Budgetary Educational Institution of Higher Professional Education "Saratov State Medical University named after. IN AND. Razumovsky" of the Ministry of Health of the Russian Federation. Statistical processing was carried out using Microsoft Office Excel 2007 (Microsoft Corp., USA) and Statistica 8.0 (StatSoft Inc., USA). The nature of the data distribution was assessed graphically and using the Shapiro-Wilk test. Descriptions of features that have a normal distribution are presented in the form M±SD, where M is the arithmetic mean, SD is the standard deviation; for features with a distribution other than normal, the results are presented in the form Ме [Q1; Q3], where Me is the median, Q1 and Q3 are the 1st and 3rd quartiles. To process data with a normal type of distribution, parametric methods were used: t-test for independent groups, paired t-test. When the data distribution was different from normal, nonparametric methods were used: Mann-Whitney test, Wald-Wolfowitz test, χ2 test, Wilcoxon test, sign test. When comparing more than two independent groups, analysis of variance methods were used: parametric analysis of variance for normally distributed data and Kruskal-Wallis rank analysis of variations for data with a non-normal distribution [34]. Results and discussion The study included 53 patients (men), their average age was 42.3±10.5 years, disease duration was 14.1±8 years (55.2%). Most patients had high disease activity: 85 (88.54%) patients had BASDAI ≥4, CRP – 12.44 [2.2; 26.2] mg/l, ESR – 18 [14; 24] mm/h. All patients noted the presence of inflammatory pain in the back (daily) and used NSAIDs for treatment. Signs of CKD were identified in 77 (80.2%) patients with SpA. A decrease in GFR in the range from 60 to 89 ml/min/1.73 m2 was detected in 39 (40.6%) patients, a decrease from 30 to 59 ml/min/1.73 m2 – in 6 (6.25%). Hyposthenuria was detected in 56 (58.33%), proteinuria – in 36 (37.5%) patients. Arterial hypertension was detected in 30 (31.25%) patients, diabetes mellitus – in 2 (2.08%). According to population studies, the incidence of CKD in the general population is 39% [CI 31; 47] [35] (differences with the incidence of CKD in patients with SpA are significant, p<0.05). The relationship between the degree of reduction in GFR and the activity of SpA has not been established. Consequently, the question arose about the relationship of the identified changes with the use of NSAIDs. The most frequently used NSAID in the examined group was nimesulide – it was taken by 70 (72.92%) patients. The average age of 35 (50%) patients (men) who used nimesulide was 41.43±10.95 years, the duration of the disease was 13.72±9.14 years. When analyzing the nimesulide dosage regimen, it was found that 18 patients had been taking the drug for 6 months. up to 1 year, 12 – from 1 year to 3 years, 22 – more than 3 and less than 10 years, 6 – more than 10 years. 8 patients take the drug in courses of 10 days several times a year, 12 – several times a week, 26 – constantly, 24 patients found it difficult to clarify the dosage regimen (they take it “on demand”). 24 patients usually take 200 mg of the drug per day, 34 – 400 mg/day, 12 – more than 500 mg/day. For severe pain, 18 (25.7%) patients take from 500 to 800 mg/day, 2 (2.85%) - more than 800 mg/day. Doses over 200 mg/day. patients took it independently, despite the recommendations of doctors. 22 (31.43%) patients noted that they exceeded the dose of 400 mg/day. several times a year, 3 (4.2%) - several times a month, 2 (2.85%) constantly violate the dosage regimen. Thus, 27 (38.57%) patients out of 70 violated the recommended regimen of drug use. 42 patients noted that they were informed by their attending physician about the possible side effects of the drug and the inadmissibility of exceeding its therapeutic dosage. 22 patients considered the ulcerogenic effect of the drug to be the most significant, 16 – hepatotoxic, 9 – a negative effect on the cardiovascular system, 2 – the ability to negatively affect hematopoiesis, 10 – the ability to disrupt kidney function. At the outpatient stage, 26 patients had their CBC and biochemical blood profile monitored once every 2-6 weeks, 16 - once every 2-6 months, 10 - once every 6-12 months, 18 - less than once a year. It should be noted that all patients combined nimesulide with proton pump inhibitors (omeprazole). An increase in liver enzymes to 2 norms was found in 4 patients, in 1 patient – to 4 norms. In patients with SpA without urinary tract pathology (UTP) and cardiovascular diseases (except for arterial hypertension stages I-II) who took nimesulide (n=66), signs of CKD were identified in 52 (78.78%) patients. An isolated decrease in GFR <89 ml/min./1.73 m2 in this group was detected in 24 (36.36%) patients, less than 60 ml/min./1.73 m2 – in 4 (6.06%). Proteinuria was detected in 23 (34.84%) patients, hyposthenuria – in 40 (60.6%). An interesting fact is that the degree of reduction in GFR was associated with the characteristics of drug use. A relationship was established between the duration of nimesulide intake and GFR (Spearman's R = -0.34, p = 0.02) (Fig. 1). At the same time, GFR in 8 patients who used courses of nimesulide several times a year and did not exceed the recommended dosage was 93.2 ml/min./1.73 m2, and a decrease in CKD of less than 89 ml/min./1.73 m2 was found in 2 patients. In 21 patients who took nimesulide several times a week and constantly at a dosage not exceeding 400 mg/day, the GFR was 84.1 ml/min./1.73 m2, a decrease in GFR from 60 to 89 ml/min./1 , 73 m2 is installed in 12 patients. In 14 patients taking the drug several times a week and constantly in a dosage exceeding 400 mg/day, SKF amounted to 68.2 ml/min./1.73 m2, and a decrease in CBP less than 89 ml/min./1.73 M2 was installed in 13 patients, of which 3 SKFs amounted to less than 60 ml/min/1.73 m2. Differences between the values of SKF patients taking nimesulide at 200-400 mg/day. and more than 400 mg/day, reliable (p <0.05). In 1 patient who took nimesulide for 3 years in a dosage of 400 mg/day. Daily, the presence of a microhematuria, stopped for 7 days, has been established. After the cancellation of the drug, SKF was 94 ml/min./1.73 m2. The analysis of the above data showed several interesting features of the application of Nimesulide in real practice. Firstly, it was established that almost every 4th patient who uses nimesulide for a long time regularly exceeds his therapeutic dosage. At the same time, patients included in the study were quite well informed about the essence of the disease obtained by the drug, possible side effects, they regularly control the UAC, the biochemical profile of the blood. Moreover, even against the background of a long administration of the drug, including In doses that exceeded the recommended, a minimum increase in the level of hepatic enzymes was noted only in 7.14% of cases. An increase in the level of enzymes has stopped within 1 week. After the cancellation of the drug. There were no serious unwanted phenomena. A retrospective analysis of the data obtained showed that the course of the drug several times a year is associated with the formation of CBP in 25% of cases (the frequency of occurrence of CBP in the population is 39% [35, 36]), a constant length of nimesulide at a dose of 400 mg/day. It is combined with the formation of stage I of HBP in no more than 40% of patients (differences with the frequency of CBP occurrence in the total population have not been established). Taking the drug in dosages exceeding the recommended leads to a more significant decrease in the severity of the CBP (before the II stadia), which is observed with a high frequency - a decrease in the SCF was established in 92.85% of patients. At the same time, the deterioration of the kidney function can be caused not only by the reception of NSAIDs, but also by persistent inflammation. The absence of the relationship between the degree of spa activity and the decrease in the SKF may be due to the fact that only patients with high activity of the disease were included in the study. Consequently, it is impossible to exclude the effect on the function of the kidneys of persistent inflammation without a study with the inclusion of patients with varying degrees of spa activity and a group of healthy individuals. Conclusion Analysis of the literature and the results of real clinical practice allows us to conclude that: 1. Most patients with spa taking nimesulide for a long time are informed by doctors about the possible side effects of the drug, regularly control the UAC, the parameters of the liver and kidney function; All patients combine the use of nimesulide with proton pump inhibitors. 2. Every 4th patient with spa regularly exceeds the recommended dosages of nimesulide. At the same time, there were no serious undesirable phenomena. 3. The degree of severity of the decrease in the SCF in patients with SPP is interconnected with the peculiarities of reception of NSAIDs (nimesulide). In patients taking the drug courses in recommended doses, the frequency of the development of CBP below the population level; In patients taking the drug constantly at a dose not exceeding 400 mg/day, the development of CBP is observed more often than with a exchange rate, but does not exceed the frequency of occurrence in the population. 4. In patients with spa, due to the pain syndrome of the long-standing nimesulide in doses exceeding the recommended therapeutic ones, the CBP is detected with a high frequency. These patients need the correction of therapy by strengthening it with biological agents or analgesics with constant control of kidney function and nephroprotective therapy. 5. The long -term reception of nimesulide in the most recommended doses is associated with minimal reversible impaired liver function in 7% of cases. Conclusion: subject to the observance of existing recommendations for the use of nimesulide (Nise®) with high efficiency, it also has a high safety profile, including with prolonged admission in patients with axial spa.
References 1. Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors // Cancer Res. 2000 Mar 1. Vol. 60 (5). R. 1306-1311. 2. Pincus T., Koch G., Lei H., Mangal B., Sokka T., Moskowitz R. et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomized, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis // Annals of the rheumatic diseases. 2004 Aug. Vol. 63(8). R. 931-939. 3. Nasonov E.L., Nasonova V.A.. Rheumatology. M.: GEOTAR-Media 2005. 4. Poddubnyy D., Haibel H., Listing J., Märker-Hermann E., Zeidler H., Braun J. et al. Influence of NSAIDs intake on the radiographic spinal progression over two years in patients with early axial spondyloarthritis // Ann Rheum Dis. 2011. Vol. 70 (Suppl 3). R. 128. 5. Zochling J., van der Heijde D., Burgos-Vargas R., Collantes E., Davis JC Jr., Dijkmans B. et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis // Ann Rheum Dis. 2006 Apr. Vol. 65(4). R. 442-452. 6. White AP, Arnold PM, Norvell DC, Ecker E., Fehlings MG Pharmacologic management of chronic low back pain: synthesis of the evidence // Spine. 2011 Oct 1. Vol. 36 (21 Suppl). R. 131-143. 7. Brattwall M., Turan I., Jakobsson J. Musculoskeletal pain: prescription of NSAID and weak opioid by primary health care physicians in Sweden 2004-2008 - a retrospective patient record review // J Pain Res. 2010. Vol. 3. R. 131-135. 8. Rebrov A.P., Gaidukova I.Z., Poddubny D.A. Cardiovascular pathology in patients with ankylosing spondylitis // Scientific and practical rheumatology. 2012. No. 51 (2). pp. 100-105. 9. Weiss P., Mouallem M., Bruck R., Hassin D., Tanay A., Brickman CM et al. Nimesulide-induced hepatitis and acute liver failure // Isr Med Assoc J. 1999 Oct. Vol. 12). R. 89-91. 10. Whelton A., Hamilton CW Nonsteroidal anti-inflammatory drugs: effects on kidney function // J Clin Pharmacol. 1991 Jul. Vol. 31(7). R. 588-598. 11. Aronoff GR Nonsteroidal anti-inflammatory drug induced renal syndromes // J Ky Med Assoc. 1992 Jul. Vol. 90 (7). R. 336-339. 12. Ejaz P., Bhojani K., Joshi VR NSAIDs and kidney // The Journal of the Association of Physicians of India. 2004 Aug. Vol. 52. R. 632-640. 13. Adam O., Vetter-Kerkhoff C., Schlondorff D. // Med Klin (Munich). 1994 Jun 15. Vol. 89(6). R. 305-311. 14. Mattia C., Ciarcia S., Muhindo A., Coluzzi F. [Nimesulide: 25 years later] // Minerva Med. 2010 Aug. Vol. 101(4). R. 285-293. 15. Rainsford KD Nimesulide — a multifactorial approach to inflammation and pain: scientific and clinical consensus // Current medical research and opinion. 2006 Jun. Vol. 22 (6). R. 1161-1170. 16. Rainsford KD Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide // Inflammopharmacology. 2006 Aug. Vol. 14 (3-4). R. 120-137. 17. Karateev A.E. Non-steroidal anti-inflammatory drugs in modern clinical practice: the pros are more than the cons // Modern Rheumatology. 2008. No. 1. P. 70-77. 18. Ward A., Brogden RN Nimesulide. A preliminary review of its pharmacological properties and therapeutic efficacy in inflammation and pain states // Drugs. 1988 Dec. Vol. 36(6). R. 732-753. 19. Aho M., Kokki H., Nikanne E. Nimesulide versus Ibuprofen for Postoperative Tonsillectomy Pain: A Double-Blind, Randomised, Active Comparator-Controlled Clinical Trial. Clin Drug Investig. 2003. Vol. 23 (10). R. 651-660. 20. Dallegri F., Ottonello L. Are there any Differences among Non-Steroidal Anti-Inflammatory Drugs? Focus on Nimesulide // Clin Drug Investig. 2007 Dec. Vol. 27. Suppl 1. R. 15-22. 21. Loh JS, Ong CW Efficacy of nimesulide versus meloxicam in the control of pain, swelling and trismus following extraction of impacted lower third molar [Int. J. Oral Maxillofac. Surg. 39 (2010) 580-584] // Int J Oral Maxillofac Surg. 2011 Jan. Vol. 40 (1). R. 125. 22. Van Steenbergen W., Peeters P., De Bondt J., Staessen D., Buscher H., Laporta T. et al. Nimesulide-induced acute hepatitis: evidence from six cases // J Hepatol. 1998 Jul. Vol. 29(1). R. 135-141. 23. Page M., Christin F., Hayi-Slayman D., Baillon JJ, Ber CE, Delafosse B. et al. // Ann Fr Anesth Reanim. 2008 Sep. Vol. 27 (9). R. 742-746. 24. Rodrigo L., de Francisco R., Perez-Pariente J.M., Cadahia V., Tojo R., Rodriguez M. et al. Nimesulide-induced severe hemolytic anemia and acute liver failure leading to liver transplantation // Scandinavian journal of gastroenterology. 2002 Nov. Vol. 37 (11). R. 1341-1343. 25. Traversa G., Bianchi C., Da Cas R., Abraha I., Menniti-Ippolito F., Venegoni M. Cohort study of hepatotoxicity associated with nimesulide and other non-steroidal anti-inflammatory drugs // Bmj. 2003 Jul 5. Vol. 327 (7405). R. 18-22. 26. Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy // The American journal of medicine. 1998 Jul 27. Vol. 105(1B). R. 31-38. 27. Laporte JR, Ibanez L., Vidal X., Vendrell L., Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents // Drug Saf. 2004. Vol. 27 (6). R. 411-420. 28. Karateev A.E., Karateev D.E., Nasonov E.L. Gastroduodenal tolerability of nimesulide (Nimesil, Berlin Chemie) in patients with a history of ulcers: the first prospective study of the safety of selective COX-2 inhibitors in patients with a high risk of developing NSAID-induced gastropathy. Scientific and Practical Rheumatology. 2003. No. 1. P. 45–48. 29. Garrett S., Jenkinson T., Kennedy LG, Whitelock H., Gaisford P., Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994 Dec. Vol. 21 (12). R. 2286-2291. 30. Rudwaleit M., Landewe R., van der Heijde D., Listing J., Brandt J., Braun J. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal // Ann Rheum Dis. 2009 Jun. Vol. 68(6). R. 770-776. 31. Rudwaleit M., van der Heijde D., Landewe R., Listing J., Akkoc N., Brandt J. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection // Ann Rheum Dis. 2009 Jun. Vol. 68(6). R. 777-783. 32. Levey AS,. Bosch JP, Lewis JB, Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group // Annals of internal medicine. 1999 Mar 16. Vol. 130(6). R. 461-470. 33. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification // American journal of kidney diseases: the official journal of the National Kidney Foundation. 2002 Feb. Vol. 39 (2 Suppl 1). R. 1-266. 34. Rebrova O.Yu. et al. Statistical analysis of medical data. Using the Statistica application package. M.: Media Sphere, 2002. 35. Clase CM, Garg AX, Kiberd BA Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) // J Am Soc Nephrol. 2002 May. Vol. 13(5). R. 1338-1349. 36. Guidelines for the Clinical Evaluation of Anti-Inflammatory and Antirheumatic Drugs (Adults and Children). Appendix 5 of the FDA. 1988.
Naisulide, 20 pcs., 100 mg, tablets
Glucocorticosteroids increase the risk of gastrointestinal ulcers or bleeding.
Antiplatelet agents and selective serotonin reuptake inhibitors (SSRIs) increase the risk of gastrointestinal bleeding.
Anticoagulants
NSAIDs may enhance the effect of anticoagulants such as warfarin. Due to the increased risk of bleeding, this combination is not recommended and is contraindicated in patients with severe coagulation disorders. If combination therapy cannot be avoided, careful monitoring of blood clotting parameters is necessary.
Diuretics
NSAIDs may reduce the effect of diuretics. In healthy volunteers, nimesulide temporarily reduces the excretion of sodium under the influence of furosemide, to a lesser extent the excretion of potassium, and reduces the diuretic effect itself. The simultaneous use of nimesulide and furosemide leads to a decrease (by approximately 20%) in the area under the concentration-time curve (AUC) and a decrease in the cumulative excretion of furosemide without changing the renal clearance of furosemide. The simultaneous use of furosemide and nimesulide requires caution in patients with renal or heart failure.
ACE inhibitors and angiotensin II receptor antagonists
NSAIDs may reduce the effect of antihypertensive drugs. In patients with mild to moderate renal failure (creatinine clearance 30-80 ml/min), with simultaneous use of ACE inhibitors, angiotensin II receptor antagonists and drugs that suppress the cyclooxygenase system (NSAIDs, antiplatelet agents), further deterioration of renal function and the occurrence of acute renal failure, which is usually reversible. These interactions should be considered in patients taking nimesulide in combination with ACE inhibitors or angiotensin II receptor antagonists.
This combination of drugs should be prescribed with caution, especially in elderly patients. Patients should be kept adequately hydrated and renal function should be closely monitored after initiating concomitant therapy.
Mifepristone
Theoretically, it is possible to reduce the effectiveness of mifepristone and prostaglandin analogues when used simultaneously with NSAIDs (including acetylsalicylic acid) due to the antiprostaglandin action of the latter.
Limited data indicate that use of an NSAID on the same day as a prostaglandin analogue does not adversely affect the effects of mifepristone or a prostaglandin analogue on cervical dilatation, uterine contractility, or reduce the clinical effectiveness of medical abortion.
The simultaneous use of nimesulide with salicylates or other non-steroidal anti-inflammatory drugs is not recommended due to the increased risk of ulceration of the gastrointestinal tract. Nimesulide displaces salicylic acid when it binds to plasma receptors.
There is evidence that NSAIDs reduce the clearance of lithium, which leads to increased plasma lithium concentrations and its toxicity. When prescribing nimesulide to patients receiving lithium therapy, the concentration of lithium in the blood plasma should be regularly monitored.
No clinically significant interactions were observed with glibenclamide, theophylline, digoxin, cimetidine and antacid drugs (for example, a combination of aluminum and magnesium hydroxides).
Nimesulide inhibits the activity of the CYP2C9 isoenzyme. When used simultaneously with nimesulide drugs that are metabolized with the participation of this enzyme, the concentration of the latter in the blood plasma may increase.
When used simultaneously with antiepileptic drugs (valproic acid), antifungal drugs (ketoconazole), antituberculosis drugs (isoniazid), amiodarone, methotrexate, methyldopa, amoxicillin in combination with clavulanic acid, an additive hepatotoxic effect is possible.
When prescribing nimesulide less than 24 hours before or after taking methotrexate, caution is required, since in such cases the concentration of methotrexate in the blood plasma and, accordingly, the toxic effects of this drug may increase.
Due to their effect on renal prostaglandins, inhibitors of prostaglandin synthetase, which include nimesulide, may increase the nephrotoxicity of cyclosporine.
In vitro studies have shown that nimesulide is displaced from the binding sites by tolbutamide and salicylic acid. Although these interactions were determined in blood plasma, these effects were not observed during clinical use of the drug.