NOOPHEN POWDER 100MG 2.5G No. 15
Instructions
Trade name of the drug: Noofen® 100;
500 (Noofen 100; 500) Active ingredients: Aminophenylbutyric acid*
Pharmacotherapeutic group: Nootropic drugs., Nootropic drugs.
Release form:
Noofen 500 powder for the preparation of a solution for oral administration. Primary packaging: powder in a laminate bag; Secondary packaging: 5 laminate bags along with instructions for use in a cardboard box. Noofen 100 powder for the preparation of a solution for oral administration. Primary packaging: powder in a laminate bag; Secondary packaging: 15 laminate bags along with instructions for use in a cardboard box.
Dosage form: Powder for solution for oral administration 100 mg 1 g N15, 500 mg 2.5 g N5 (sachets)
Compound:
Each sachet contains 100 mg -amino--phenylbutyric acid hydrochloride. Each sachet contains 500 mg -amino--phenylbutyric acid hydrochloride. Excipients: mannitol, aspartame, Orange Durarome flavoring.
Pharmacological properties:
Noofen is a psychometabolic stimulant that has a beneficial effect on metabolic processes in the nerve cells of the brain. The active substance of the drug Noofen can be considered as a derivative of β-aminobutyric acid (GABA) or as a derivative of β-phenylethylamine. Noofen has nootropic activity, and as a GABA derivative it has an anxiolytic (tranquilizing) effect. The drug reduces anxiety, restlessness, fear and improves sleep, so it is prescribed for the treatment of neuroses and before operations. Noofen prolongs and enhances the effect of hypnotics, narcotics, neuroleptics and antiparkinsonian drugs, but does not have an anticonvulsant effect. Noofen lengthens the latent period of nystagmus and shortens its duration and severity. The drug significantly reduces the manifestations of asthenia and vasovegetative symptoms, including headache, a feeling of heaviness in the head, sleep disturbance, irritability, emotional lability and increases mental performance. Psychological indicators (attention, memory, speed and accuracy of sensory-motor reactions) improve under the influence of the drug Noofen. In patients with asthenia and in emotionally labile patients, from the first days of therapy, subjective well-being improves, interest and initiative, and motivation for activity increase, without causing unnecessary sedation or agitation. The drug is well absorbed from the gastrointestinal tract and penetrates all tissues of the body, easily crossing the blood-brain barrier. Metabolized in the liver to pharmacologically inactive metabolites. About 5% is excreted unchanged by the kidneys. The drug does not accumulate in the body.
Indications for use:
Asthenic and anxious-neurotic states: restlessness, fear, anxiety; obsessive-compulsive neurosis, psychopathy; in older people – insomnia, restlessness at night. Prevention of stress before operations or painful diagnostic tests. Meniere's disease, prevention of dizziness associated with dysfunction of the vestibular analyzer of various origins. - Prevention of kinetosis. In children – treatment of stuttering and tics. An auxiliary agent in complex treatment for the relief of alcohol withdrawal syndrome (psychopathological and somatovegetative disorders).
Mode of application:
The contents of the sachet are dissolved in half a glass of boiled water and taken orally before meals. Asthenic and anxiety-neurotic conditions: adults – 500 mg (1 sachet of Noofen 500) 1-3 times a day. If necessary, the daily dose can be increased to 2.5 g (5 sachets). The course of treatment is 2-3 weeks. If necessary, the course can be extended to 4-6 weeks. Meniere's disease and dizziness of various origins associated with dysfunction of the vestibular analyzer: - dysfunction of the vestibular analyzer of infectious origin and during an exacerbation of Meniere's disease, 500 mg 4 times a day for 5-7 days, with a decrease in the severity of vestibular disorders - 500 mg of the drug 2-3 times a day for 5-7 days, then 500 mg 1 time a day for another 5 days. For a relatively mild course of the disease, you should take 500 mg once a day for 5-7 days, then continue treatment by taking a Noofen 100 mg bag 2-3 times a day for 7-10 days. - dysfunction of the vestibular analyzer of vascular and traumatic origin, 500 mg 1-2 times a day for 12 days. To prevent kinetosis (motion sickness), 500 mg is prescribed once, one hour before the expected start of motion sickness or when the first symptoms of seasickness appear. When severe symptoms occur (vomiting, etc.), taking the drug is ineffective. An auxiliary remedy for the relief of alcohol withdrawal syndrome: in the first days, take 500 mg 2-3 times during the day and 500 mg at night, with a gradual decrease in the daily dose to the usual one. For complex treatment of severe vertebral pain syndrome against the background of menopausal disorders: 500 mg 2 times a day for the first two weeks, the next two weeks - 500 mg daily. In case of moderate severity of pain and menopausal disorders, it is recommended to use Noofen 500 at a dose of 500 mg daily for 4 weeks. There is no addiction or dependence on the drug, there is no “withdrawal syndrome”. Noofen 100 powder for the preparation of a solution for oral administration: The contents of the sachet are dissolved in half a glass of boiled water and taken orally before meals. Children from 3 to 4 years old are prescribed 100 mg (1 sachet) 2 times a day, from 5 to 6 years old - 100 mg 2-3 times a day, from 7 to 10 years old. 10 years old - 100 mg 3-4 times a day, from 11 to 14 years old - 200 mg (2 sachets) 2-3 times a day. For children over 14 years of age - doses for adults (the use of Noofen 500 powder or other dosage forms of Noofen is recommended). Highest single doses for children: from 3 to 6 years – 100 mg, from 7 to 10 years – 200 mg, from 11 to 14 years – 300 mg. There is no addiction or dependence on the drug, there is no “withdrawal syndrome”.
Side effects:
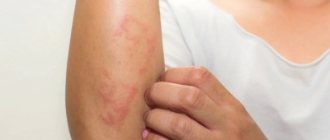
Like other medicines, Noofen can cause side effects, although not everyone gets them. Noofen is usually well tolerated. Nervous system disorders: drowsiness* (at the beginning of treatment). Gastrointestinal disorders: nausea* (at the beginning of treatment). Disorders of the skin and subcutaneous tissues: rarely - allergic reactions (skin rash, itching). Disorders of the liver and biliary tract: with long-term use of high doses - hepatotoxicity*. * frequency of manifestation is unknown (cannot be determined from available data). If you experience any side effects that are not listed in these instructions, or if any of the side effects mentioned are particularly severe, please consult your doctor.
Contraindications:
- Hypersensitivity to the active substance or excipients of the drug. - Pregnancy and breastfeeding. Children's age up to 3 years. Noofen 100 and Noofen 500 powders contain the sweetener aspartame. Its use is contraindicated in patients with phenylketonuria.
Drug interactions:
Noofen can be combined with psychotropic drugs, reducing the dose of Noofen and the drugs combined with it. Noofen enhances and prolongs the effect of hypnotic, antipsychotic and antiparkinsonian drugs.
Special instructions:
Caution should be exercised in patients with erosive and ulcerative lesions of the stomach and/or duodenum. To protect the mucous membrane from the irritating effect of the drug, these patients are prescribed smaller doses. With long-term use, it is necessary to monitor peripheral blood counts and liver function tests. Patients with renal and/or liver failure during long-term use should monitor liver and/or kidney function. Effect on the ability to drive Patients who experience drowsiness or other disorders of the central nervous system while using the drug should be careful when driving or servicing machinery during treatment. The drug should not be used after the expiration date and should be stored out of the reach of children. Use during pregnancy and breastfeeding is contraindicated.
Overdose:
There have been no reports of overdose cases. The drug is low toxic. Symptoms: drowsiness, nausea, vomiting, dizziness. With long-term use of high doses, eosinophilia, arterial hypotension, fatty liver (taking more than 7 g), and impaired renal function may develop. Treatment: gastric lavage, taking activated charcoal, supportive and symptomatic treatment. A specific antidote is not known.
Storage conditions: Store in a dry place at a temperature not exceeding 25ºС.
Shelf life: 2 years
Dispensing conditions: Without a prescription
Noofen 100 1 g No. 15 portion size for oral administration
Instructions for medical use of the drug NOOFEN 100 Trade name Noofen 100 International nonproprietary name No Dosage form Powder for the preparation of solution for oral administration, 100 mg Composition One sachet contains the active substance - phenibut (g-amino-b-phenylbutyric acid hydrochloride) 100 mg. excipients: mannitol, aspartame, Orange Durarome flavoring. Description Powder from white to light cream color. Inclusions of yellow color are allowed. Pharmacotherapeutic group Psychoanaleptics. Psychostimulants and nootropics. Other psychostimulants and nootropics. ATX code N06BX Pharmacological properties Pharmacokinetics The drug is well absorbed after oral administration and penetrates all tissues of the body, passes well through the blood-brain barrier. Distribution in the liver and kidneys is close to uniform, while in the brain and blood it is less uniform. After 3 hours, a noticeable amount of the administered drug is found in the urine, while the concentration of the drug in the brain tissue does not decrease; it is detected in the brain after another 6 hours. The next day, the drug can only be detected in the urine; it is found in the urine 2 days after administration, but the amount detected is only 5% of the administered dose. The greatest binding of the drug occurs in the liver (80%), it is not specific. With repeated administration, cumulation is not observed. Pharmacodynamics The active substance Noofen 100 is a derivative of g-aminobutyric acid (GABA) and phenylethylamine. Its antihypoxic and antiamnestic effects are dominant. Noofen 100 has nootropic activity, and as a derivative of GABA it has a tranquilizing effect, stimulates learning processes and improves memory, increases physical performance, relieves stress, anxiety, fear and improves sleep, prolongs and enhances the effect of hypnotics, narcotics, antipsychotics and anticonvulsants. Does not affect cholinergic and adrenergic receptors. Noofen 100 lengthens the latent period and shortens the duration and severity of nystagmus. Noofen 100 significantly reduces the manifestations of asthenia and vasovegetative symptoms, including headache, a feeling of heaviness in the head, sleep disturbance, irritability, emotional lability and increases mental performance. Mental indicators (attention, memory, speed and accuracy of sensory-motor reactions) improve under the influence of Noofen, in contrast to the influence of tranquilizers. In patients with asthenia and in emotionally labile patients, from the first days of therapy, their well-being improves, interest and initiative increase, and motivation for active activity without sedation or agitation. It has been established that Noofen 100, used after a traumatic brain injury, increases the number of mitochondria and improves the bioenergetics of the brain. Noofen 100 normalizes lipid peroxidation processes. Indications for use In children - asthenic and anxious-neurotic conditions: restlessness, fear, anxiety; obsessive-compulsive neurosis, psychopathy - treatment of stuttering, enuresis, tics - prevention of stressful conditions, before operations or painful diagnostic tests - dizziness associated with dysfunction of the vestibular apparatus of various origins, for the prevention of motion sickness Method of administration and dosage The contents of the sachet are dissolved in half a glass of warm water and taken orally before meals. The course of treatment is 2-6 weeks. Children from 3 to 4 years old are prescribed 100 mg (1 sachet) 2 times a day, from 5 to 6 years old - 100 mg 2-3 times a day, from 7 to 10 years old. 10 years old - 100 mg 3-4 times a day, from 11 to 14 years old - 200 mg (2 sachets) 2-3 times a day. Children over 14 years of age are prescribed adult doses of Noofen 250 mg tablets, Noofen 250 mg capsules or Noofen 500 mg powder for oral solution. Highest single doses for children: up to 6 years – 100 mg, from 7 to 10 years – 200 mg, from 11 to 14 years – 300 mg. If you miss a dose, take the drug as soon as you remember, but skip it if it is almost time for your next dose. Never take double doses. Side effects Noofen 100 is usually well tolerated. - drowsiness, nausea, increased irritability, agitation, anxiety, headache, dizziness (after the first doses) - allergic reactions (skin rash, itching) sometimes - hepatotoxicity (with long-term use of high doses) If you experience any side effects, which are not listed in this instruction, or any of the side effects mentioned are particularly severe, please consult a doctor. Contraindications - hypersensitivity to the active substance or excipients of the drug - phenylketonuria (Noofen 100 powder contains the sweetener aspartame) Drug interactions Prolongs and enhances the effects of sleeping pills, narcotic analgesics, antiepileptic and antiparkinsonian drugs, tranquilizers and antipsychotics. Special instructions Caution should be exercised in patients with erosive and ulcerative diseases of the gastrointestinal tract due to the irritating effect of the drug. These patients are prescribed smaller doses of the drug. With long-term use, it is necessary to monitor liver function indicators and peripheral blood patterns. Pediatrics The use of the drug in children under 3 years of age has not been studied. Overdose of Noofen 100 is a low-toxic drug; only in a daily dose of 7-14 g with long-term use it can be hepatotoxic. These doses significantly exceed the recommended dose (the average therapeutic dose is 500 – 2500 mg). Eosinophilia and fatty liver were observed only at the highest dose used. There were no such changes when using the drug in lower doses. Symptoms: drowsiness, nausea, vomiting, eosinophilia, decreased blood pressure. Treatment: gastric lavage. Therapy is symptomatic. In case of complications (arterial hypotension, renal failure), auxiliary and symptomatic measures are used. If you suspect an overdose, you should call a doctor. Release form and packaging 1 g mg powder in a laminated bag. 15 sachets each along with instructions for medical use in the state and Russian languages in a cardboard pack. Storage conditions Store in a dry place at a temperature not exceeding 25 °C. Keep out of the reach of children. Shelf life: 2 years The drug cannot be used after the expiration date. Conditions for dispensing from pharmacies According to prescription Manufacturer: OLAINPHARM JSC. Address: st. Rupnitsa 5, Olaine, LV - 2114, Latvia. Owner of the registration certificate LLC "OLFA" Address: st. Dankevicha 4, Kyiv, 02232, Ukraine. Address of the organization receiving claims from consumers regarding product quality in the territory of the Republic of Kazakhstan: 050009 Almaty, 151/115 Abay Ave., office 807 Telephone / fax 007 E-mail
Noofen tablets 250 mg No. 10x2
Name
Noofen tablet 250 mg in container pack No. 10x2
Main active ingredient
Gamma-amino-beta-phenylbutyric acid hydrochloride
Release form
Pills
Compound
Active ingredient: α-amino-β-phenylbutyric acid hydrochloride 250 mg. Excipients: lactose monohydrate, potato starch (E 1401), calcium stearate (E 572).
Description
Flat-cylindrical tablets from white to white with a slight yellowish tint, with a chamfer and a score on one side of the tablet.
Dosage
250mg
Pharmacological properties
Pharmacodynamics
The active substance Noofen (?-amino-?-phenylbutyric acid hydrochloride) can be considered as a derivative of ?-aminobutyric acid (GABA) or as a derivative of ?-phenylethylamine. Noofen has nootropic activity, and as a GABA derivative it has an anxiolytic (tranquilizing) effect. Does not affect cholinergic and adrenergic receptors. Noofen does not have anticonvulsant activity. Noofen lengthens the latent period of nystagmus and shortens its duration and severity.
Pharmacokinetics
The drug is well absorbed after oral administration and penetrates all tissues of the body, overcomes the blood-brain barrier. About 0.1% of the administered dose of the drug penetrates into the brain tissue, and to a much greater extent in young and elderly people. Metabolized in the liver - 80-95%, to pharmacologically inactive metabolites. About 5% is excreted unchanged by the kidneys. With repeated administration, cumulation is not observed.
Indications for use
Used for increased nervous excitability (neurasthenia) and sleep disorders. In children - for the treatment of stuttering and tics.
Contraindications
Hypersensitivity to the active substance or excipients of the drug. Acute renal failure. During pregnancy and breastfeeding.
Use during pregnancy and lactation
Use during pregnancy and breastfeeding is contraindicated, because There are no adequate and well-controlled clinical studies on the safety of the drug during these periods. Experimental studies on animals did not establish the mutagenic, teratogenic and embryotoxic effects of the drug.
Impact on the ability to drive vehicles and maintain machinery
Patients who experience drowsiness, dizziness or other disorders of the central nervous system should not drive vehicles or operate machinery during treatment.
Directions for use and doses
Noofen tablets are taken orally after meals with water. The duration of the course of treatment is determined by the doctor taking into account the disease, tolerability of the drug and the achieved effect. The tablet can be divided into two equal doses. For increased nervous excitability (neurasthenia) and sleep disorders, 250-500 mg 3 times a day. Highest single doses: for adults - 750 mg, for persons over 60 years old - 500 mg. The course of treatment is 2-3 weeks. If necessary, the course can be extended to 4-6 weeks. Use in children and adolescents (for the treatment of stuttering, tics) For children under 8 years of age, the dose is 125 mg 3 times a day, for children aged 8 to 14 years - 250 mg 3 times a day. Children over 14 years of age are prescribed adult doses. The use of tablet dosage forms in children under 6 years of age is accompanied by an increased risk of aspiration, since full control of the swallowing reflex develops by the age of six. For use in children under 6 years of age, it is recommended to prepare the powder or suspension in a pharmacy for dosing accuracy. Patients with impaired liver function In patients with impaired liver function, high doses of Noofen may cause a hepatotoxic effect. Patients in this group are prescribed smaller doses of the drug under monitoring of liver function. Patients with impaired renal function The use of the drug is contraindicated in patients with acute renal failure. With long-term use in patients with impaired liver and/or kidney function, it is necessary to monitor indicators of kidney and liver function. If you miss a dose, take the medicine as soon as you remember, but skip it if it is almost time for your next dose. Never take double doses to replace a missed dose.
Side effect
Noofen, like other medicines, can cause side effects that do not occur in all patients. Classification of undesirable side reactions by frequency of development: very often (? 1/10); often (? 1/100 to
Overdose
There have been no reports of overdose cases. Symptoms: drowsiness, nausea, vomiting, dizziness. With long-term use of high doses, eosinophilia, arterial hypotension, renal impairment, and fatty liver may develop (taking more than 7 g). Treatment: gastric lavage, symptomatic treatment. There is no specific antidote.
Interaction with other drugs
Combining the drug Noofen with other psychotropic drugs requires prior consultation with a doctor and observation by a doctor during the treatment process. The simultaneous administration of Noofen with carbamazepine, oxcarbazepine or MAO inhibitors is not recommended.
Precautionary measures
Caution should be exercised in patients with erosive and ulcerative diseases of the gastrointestinal tract due to the irritating effect of the drug. These patients are prescribed smaller doses of the drug. With long-term use, it is necessary to monitor peripheral blood parameters and liver function. If symptoms of the disease persist or worsen while taking the drug, you should consult a doctor. Noofen 250 mg tablets contain lactose. Should not be used in patients with congenital galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C. Keep out of the reach of children.