Indoxyl
Use during pregnancy and breastfeeding
Pregnancy
There have been no controlled studies in pregnant women using a topical gel containing a combination of clindamycin and benzoyl peroxide.
Data on the use of topical preparations containing one of the active ingredients (clindamycin or benzoyl peroxide) in pregnant women are limited. Preclinical studies do not confirm direct or indirect adverse effects that can be attributed to reproductive toxicity.
Given the low level of systemic exposure to clindamycin and benzoyl peroxide, adverse effects on pregnancy are unlikely. However, the combination of clindamycin and benzoyl peroxide should be used during pregnancy only if the expected benefit to the mother outweighs the potential risk to the fetus.
Lactation
There is no data on the safety of topical use of the combination of clindamycin and benzoyl peroxide during breastfeeding.
Clindamycin and benzoyl peroxide have poor percutaneous absorption, but there is no data on the excretion of clindamycin or benzoyl peroxide into breast milk when administered topically. Clindamycin is excreted into breast milk when administered orally and parenterally.
The combination of clindamycin and benzoyl peroxide should be used during lactation only if the expected benefit to the mother outweighs the potential risk to the baby.
To avoid accidental entry of the drug into the child’s body when using it during lactation, the drug should not be applied to the skin of the breast.
Use for liver dysfunction
There is no need for dose adjustment in patients with liver failure.
Because the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is poor, hepatic impairment is unlikely to contribute to clinically significant systemic exposure.
Use for renal impairment
There is no need for dose adjustment in patients with renal failure.
Because the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is poor, renal impairment is unlikely to contribute to clinically significant systemic exposure.
Use in children
Contraindicated for children under 12 years of age. The safety and effectiveness of the combination of clindamycin and benzoyl peroxide have not been studied in children under 12 years of age, and therefore the use of the combination of clindamycin and benzoyl peroxide is not recommended in this population.
Use in elderly patients
There are no special recommendations for elderly patients.
special instructions
Avoid getting the drug into the mouth, eyes, lips, other mucous membranes, as well as irritated damaged areas of the skin. If the gel accidentally gets into these areas, they should be rinsed well with water. During the first week of treatment, most patients experience increased peeling and redness of the skin. Depending on the severity of these side effects, patients may use moisturizers, temporarily reduce the frequency of use of the drug, or temporarily discontinue use. The effectiveness of the drug when used less than once a day has not been studied.
If severe skin irritation occurs (eg, severe erythema, severe skin dryness and itching, severe tingling/burning), use of the drug should be discontinued.
Since benzoyl peroxide may cause increased sensitivity to sunlight, you should avoid prolonged exposure to sunlight and do not visit a solarium while using the drug. If sun exposure cannot be avoided, you should use sunscreen cosmetics for your skin and clothing that protects you from the sun's rays.
If the patient has sunburn, you should stop using the drug until the burns are healed.
The drug can discolor hair and colored fabric. Avoid contact of the drug with hair, fabric, furniture or carpeting.
Pseudomembranous colitis
Cases of pseudomembranous colitis have been reported with the use of almost all antibacterial agents, including clindamycin. Their severity varies in severity from mild to life-threatening. Pseudomembranous colitis may occur several weeks after discontinuation of therapy.
Although pseudomembranous colitis is unlikely to occur with topical use of a combination of clindamycin and benzoyl peroxide, if prolonged or severe diarrhea occurs or if the patient suffers from abdominal cramps, treatment should be stopped immediately and a doctor should be consulted, as symptoms may indicate the presence of colitis caused by pseudomembranous colitis. taking an antibiotic.
Resistance to clindamycin
Benzoyl peroxide reduces the likelihood of the emergence of microorganisms resistant to clindamycin. However, patients who have recently used parenteral or topical clindamycin or erythromycin are more likely to have propionobacteria and saprophytic flora with previously acquired antimicrobial resistance.
Cross resistance
The presence of cross-resistance between clindamycin and lincomycin has been established.
Resistance to clindamycin is often associated with resistance to erythromycin.
Impact on the ability to drive vehicles and operate machinery
A negative effect of the drug on the ability to drive vehicles or operate machinery is unlikely.
Introduction
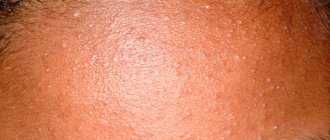
Acne is a multifactorial chronic dermatosis that affects up to 83–85% of teenage girls and 95–100% of teenage boys [1].
In addition, acne may persist or occur in adults. Acne negatively affects the patient's physical condition, and can also be a source of social problems and pharmacoeconomic consequences [2, 3]. Acne is one of the most common dermatoses. According to the literature, up to 650 million people suffer from acne in different countries [4], incl. about 90% of the teenage population of Europe [3, 5]. At the same time, acne is increasingly being reported in adults. Thus, in a study conducted in the UK with the participation of 749 adult volunteers over 25 years of age, the prevalence of facial acne in women was 54%, in men – 40% [6].
A survey of 197 Japanese schoolchildren and students showed that from primary school to college age, acne occurred in 58.6% of cases, and 93.3% of university students encountered this disease at least once during their lives [7]. In China, among 17,345 surveyed, 1.6% of adolescents aged 10 years suffered from acne; as age increased, the incidence of the disease increased and reached 46.8% at the age of 19 years, and decreased to 15.8% at the age of 25–29.
The authors emphasize that among adolescents 17–20 years old, acne is most common among men, and at the age of 30 years, it is more common among women. In general, after 25 years, facial acne is observed in approximately 50% of cases; in 12% of women and 3% of men, their clinical manifestations persist until middle age [8]. There is also an increase in the prevalence of acne among women. According to S. Preneau et al., this disease affects up to 50% of women over 25 years of age [9].
Pathogenesis
An important factor in the occurrence of acne is inflammation of the sebaceous follicle, which is the result of a combination of genetic predisposition (increased size and number of sebaceous glands), hormonal levels (androgens), exposure to bacteria (Propionbacterium acnes) and the inflammatory reaction of the hair follicle and sebaceous gland. Androgens stimulate increased production of sebum in the sebaceous glands; bacteria contain lipase, which converts lipids into fatty acids. Excess sebum and fatty acids cause corneocytes to block the hair follicle and sebaceous gland. As a result, the primary focus of acne is formed - a comedon [10].
Clinic and course
Acne is localized mainly on the skin of the face, upper extremities, upper chest and back and manifests itself as papules, pustules and nodules, as well as open and closed comedones [11].
The primary focus of acne, microcomedone, is a collection of exfoliated corneocytes in the proximal zone of the pilosebaceous follicle. Its evolution proceeds through further hyperproliferation, leading to the accumulation of corneocytes and sebum in the lumen of the follicle and the formation of a “plug”, clinically manifested by an open or closed comedon, or along the path of inflammation caused by the diffusion of soluble components of the comedon into the dermis with the formation of papules, pustules, nodes and cysts .
Closed comedones (white heads) present as pale, slightly raised, pinpoint papules without a clinically visible opening and are difficult to visualize. Open comedones can be flat or slightly raised above the skin level; their black color is due to melanin and oxidized lipids deposited within the detritus.
Erythematous papules have a diameter of 1–5 mm. The pustules are approximately the same size, filled with white sterile pus, and as the pathological process progresses, they turn into painful, dense nodes. Cystic acne elements are located deeper and are filled with pus and serous-bloody fluid [12].
More severe acne more often affects men [13].
Acne in children occurs during adolescence, which is characterized by serious physiological changes in the body. It is no coincidence that the manifestation of the comedonal form of acne, in particular in the central part of the face, is often the first sign of puberty. During puberty, an important factor in the pathogenesis of acne is the increased production of sebaceous gland secretions, due to their androgenic stimulation.
Mild acne often occurs in early adolescence and is limited to the appearance of comedones in the central part of the face. Their regression is often accompanied by the risk of hyperpigmentation and the formation of pinpoint, atrophic and hypertrophic scars in their place [13].
In adults, acne can persist from adolescence or first appear after age 25 and is usually mild to moderate in nature, affecting mainly the face.
There are two clinical forms of the disease:
- the most common inflammatory form, manifested by papulopustular elements and nodules on the skin of the lower part of the face;
- a less common retention form, characterized by the formation of “black dots” in the form of microcysts in combination with hyperseborrhea.
It is believed that the occurrence of acne in adults is due to hormonal causes, the presence of antibiotic-resistant strains of bacteria, the use of cosmetics, medications, smoking, chronic stress, and genetic factors. Confirmation of the important role of the latter is the presence in almost 50% of patients with acne of a first-degree relative with acne who developed after adolescence.
Thus, acne in adults differs from teenage acne: among adolescents, the disease is more common in young men, and they have the most severe forms of the disease, while after adolescence, acne affects mainly women and has a more pronounced inflammatory nature, and comedones, like usually absent or rare [9, 14, 15].
Taking into account the clinical picture and course of the disease, as well as the development of scars, acne is classified into mild, moderate and severe degrees of severity. Mild degree is characterized by the presence of comedones (white heads and/or blackheads), a small number of papules and pustules (usually less than 10) and the absence of nodes.
Moderate degree is characterized by the presence of several or numerous papules and pustules (from 10 to 40) and comedones (from 10 to 40). The presence of more than 40 papules and pustules with more extensive and deep nodular inflammatory elements (up to 5) is also classified as moderate acne.
In severe cases, there are numerous papules and pustules, as well as multiple (more than 5) nodular elements and scars that form as a result of previous severe acne [15].
Treatment
According to European (EADV) and Russian (RODVK) clinical guidelines, the choice of the optimal treatment option for acne should be made taking into account the clinical form and severity of the disease. It may also depend on the patient's quality of life indicators. In this case, it is advisable to prescribe more aggressive therapy to patients with a pronounced decrease in quality of life [11, 16].
For mild to moderate papulopustular acne, the high-level evidence recommendations are a fixed-dose combination of benzoyl peroxide and adapalene and a fixed-dose combination of benzoyl peroxide and clindamycin. Moderate-level evidence recommendations include the use of azelaic acid, benzoyl peroxide, topical retinoids, a combination of a systemic antibiotic, and adapalene. For moderate, more widespread forms of the disease, low-level evidence recommendations include blue light monotherapy, a fixed-dose combination of isotretinoin and erythromycin, oral zinc, a combination of a systemic antibiotic with either benzoyl peroxide or adapalene as a fixed-dose combination with benzoyl peroxide (for moderate , a more widespread form of the disease). Monotherapy with topical antibiotics is not recommended (i.e. has a negative recommendation) for the treatment of mild to moderate forms of papulopustular acne.
For nodular/globular acne, the recommendation of a high level of evidence is monotherapy with oral isotretinoin (conglobate form); recommendation of moderate level of evidence - a combination of a systemic antibiotic and azelaic acid (conglobate form); low level of evidence recommendation – oral antiandrogen drugs and oral antibiotics (conglobate form); a combination of a systemic antibiotic and adapalene, benzoyl peroxide and a fixed dose combination of adapalene/benzoyl peroxide [11, 16].
The presented treatment options affect various pathophysiological factors in the development of acne.
When choosing the optimal treatment option for a particular patient, taking into account the severity of his disease, doctors need to find a drug with an appropriate balance of characteristics such as effectiveness, onset of action and the risk of side effects.
In this regard, you should pay attention to a drug with a high level of evidence from European, Russian clinical guidelines and the American Academy of Dermatology (2015) for mild to moderate acne, in particular for inflammatory lesions in adults and adolescents aged 12 years and older, use of Indoxyl gel 5% once a day [11, 16–19].
Below is a brief description of the effectiveness and tolerability of Indoxyl gel 5% for use once a day for acne.
Indoxyl (clindamycin phosphate + benzoyl peroxide) is a registered trademark of Stifel, part of the Glaxo Smith Kline (GSK) group of companies.
According to the results of clinical studies, the use of Indoxyl gel (clindamycin 1% + benzoyl peroxide 5%) once a day compared with combination therapy with adapalene 0.1% + benzoyl peroxide 2.5% demonstrates:
- more significant therapeutic effect - after 12 weeks of treatment in the clindamycin 1% + benzoyl peroxide 5% group, the therapeutic effect was recorded in a statistically significantly larger number of patients (improvement by more than 2 points on the Investigator's Global Statistical Assessment [ISGA] scale relative to baseline values) compared with patients in the adapalene 0.1% + benzoyl peroxide 2.5% group (p<0.05);
- faster onset of action - in the clindamycin 1% + benzoyl peroxide 5% group, the time to achieve a therapeutic effect was statistically significantly shorter than in the adapalene 0.1% + benzoyl peroxide 2.5% group (p < 0.05), after 4 weeks in the group of clindamycin 1% + benzoyl peroxide 5%, a statistically significantly more pronounced decrease in the number of inflammatory elements was registered than in the group of adapalene 0.1% + benzoyl peroxide 2.5% (p < 0.05);
- comparable tolerability profile - in the group of adapalene 0.1% + benzoyl peroxide 2.5% at all analyzed time points, starting from the 1st week, erythema, dryness, peeling, itching and burning/tingling sensation were recorded more often (p<0.05) .
Treatment-related side effects were reported in 48.4% of patients in the clindamycin 1% + benzoyl peroxide 5% group and 78% of patients in the adapalene 0.1% + benzoyl peroxide 2.5% group [18].
Conclusion
Thus, in terms of clinical effectiveness, timing of clinical effect, treatment tolerability and improvement in the patient’s quality of life, the use of Indoxyl gel (a combination of clindamycin 1% and benzoyl peroxide 5%) turned out to be optimal compared to combination therapy with adapalene 0.1% + benzoyl peroxide 2, 5% for both immediate and long-term results.
Indoxyl 50 mg+10 mg/g gel for external use 25 g
Description
Gel for external use is white to yellowish in color, homogeneous.
Compound
1 g
benzoyl peroxide 66.7 mg,
which corresponds to the content of anhydrous benzoyl peroxide* 50 mg
clindamycin phosphate** 12.8 mg,
which corresponds to the content of clindamycin* 10 mg
* the amounts of active ingredients may be adjusted depending on the purity of the series of substances.
** in the production of clindamycin, phosphate is added with a 2% excess.
Excipients: carbomer - 20 mg, dimethicone - 1 mg, disodium lauryl sulfosuccinate - 0.4 mg, disodium edetate - 1 mg, glycerol - 40 mg, hydrated colloidal silicon dioxide - 2.5 mg, poloxamer 182 - 2 mg, sodium hydroxide - 3.1 mg, purified water - up to 1000 mg.
Package
15 g - aluminum tubes (1) - cardboard packs.
25 g - aluminum tubes (1) - cardboard packs.
pharmachologic effect
Mechanism of action
Clindamycin is an antibiotic of the lincosamide group with a bacteriostatic effect against gram-positive aerobic microorganisms and a wide range of anaerobic bacteria. Lincosamides, such as clindamycin, bind to the 50S ribosomal subunit of susceptible bacteria and, by interfering with eptidyl transport, prevent elongation of peptide chains. The action of clindamycin is mainly bacteriostatic, however, high concentrations may have a slow bactericidal effect against susceptible strains.
Although clindamycin phosphate is inactive in vitro, rapid hydrolysis in vivo converts this compound to active clindamycin.
Benzoyl peroxide is a highly lipophilic oxidizing agent with a bactericidal and weak keratolytic effect. Benzoyl peroxide has a nonspecific bactericidal mechanism of action, producing reactive oxygen species that prevent the emergence of clindamycin-resistant microorganisms.
Pharmacodynamic effects
Clindamycin is active in vitro against Propionibacterium acnes, which causes acne. There is evidence of the possibility of P. acnes developing resistance to clindamycin. Clindamycin inhibits P. acnes in vitro (minimum inhibitory concentration (MIC) 0.4 μg/ml). Once applied, clindamycin reduces free fatty acids on the skin surface from approximately 14% to 2%. Clindamycin reduces inflammation by inhibiting leukocyte chemotaxis.
The effectiveness of benzoyl peroxide in the treatment of acne is mainly attributed to its bactericidal activity, especially against P. acnes. The bactericidal activity of benzoyl peroxide is caused by the release of active or free oxygen radicals that can oxidize bacterial proteins. Benzoyl peroxide is effective in the treatment of acne due to its anti-inflammatory and some keratolytic effects.
Resistance and cross-resistance
Treatment of acne with topical and oral antibiotics such as clindamycin and erythromycin as monotherapy has been associated with the development of antimicrobial resistance in P. acnes as well as in saprophytic flora (eg, Staphylococcus aureus, Streptococcus pyogenes). Long-term use of clindamycin may cause the development of resistance in these microorganisms.
Benzoyl peroxide has a bactericidal effect and has not caused resistance in P. acnes. The inclusion of benzoyl peroxide in a combination formulation containing 1% clindamycin and 5% benzoyl peroxide reduced the number of clindamycin-resistant P. acnes organisms.
The level of acquired resistance for specific organisms may vary depending on geographic location and time.
Where possible, local resistance data should be taken into account, particularly when treating serious infections.
The presence of cross-resistance between clindamycin and lincomycin has been established.
Pharmacokinetics
Absorption, distribution and metabolism
Clindamycin phosphate undergoes rapid hydrolysis by skin phosphatases and is converted to clindamycin. Clindamycin is then metabolized to clindamycin sulfoxide. Significant concentrations of clindamycin were found in acne lesions in patients who used topical clindamycin phosphate for 2 weeks.
There is no evidence of systemic accumulation of clindamycin or accumulation of clindamycin in the skin after repeated use.
Clindamycin is metabolized in the liver to active and inactive metabolites.
Benzoyl peroxide is absorbed by the skin and metabolized to benzoic acid. When applied topically, less than 5% of the dose in the form of benzoic acid enters the systemic circulation.
The presence of benzoyl peroxide does not affect the transdermal absorption of clindamycin.
Removal
Clindamycin is excreted primarily in the urine in the form of the parent substance. T1/2 of clindamycin is about 9 hours. After repeated topical application of a gel containing clindamycin, less than 0.06% of the dose was excreted in the urine.
Benzoyl peroxide is excreted in the urine in the form of benzoic acid.
Indications
- acne (acne vulgaris) of mild to moderate severity, especially with a predominance of inflammatory skin lesions.
Dosage regimen
Indoxyl is intended for topical use only.
The drug should be applied in a thin layer to the entire affected area 1 time/day after thoroughly cleansing the skin with a mild cleanser and completely drying the skin.
The gel is poorly absorbed if an excessive amount of the drug is applied to the skin. After applying the gel, you should wash your hands.
If necessary, you can use a moisturizer after the gel is absorbed.
If dry skin or flaking occurs, you should reduce the frequency of use of the gel or temporarily suspend treatment. The effectiveness of the drug when used less than once a day has not been studied.
Applying the gel more frequently than recommended by the instructions does not increase the effectiveness of treatment, but may increase the risk of skin irritation.
The duration of treatment required to achieve a therapeutic effect is from 2 to 5 weeks, but not more than 12 weeks.
The safety and effectiveness of the combination of clindamycin and benzoyl peroxide in the treatment of acne lasting more than 12 weeks have not been studied. The expected benefit to the patient should be carefully assessed when prescribing treatment for more than 12 weeks of continuous use.
Special patient groups
The safety and effectiveness of the combination of clindamycin and benzoyl peroxide have not been studied in children under 12 years of age, and therefore the use of the combination of clindamycin and benzoyl peroxide is not recommended in this population.
There are no special recommendations for elderly patients.
In patients with renal failure, there is no need for dose adjustment. Because the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is poor, renal impairment is unlikely to contribute to clinically significant systemic exposure.
In patients with liver failure, there is no need for dose adjustment. Because the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is poor, hepatic impairment is unlikely to contribute to clinically significant systemic exposure.
Side effect
The adverse events presented below are listed according to the damage to organs and organ systems and the frequency of occurrence. The frequency of occurrence is defined as follows: very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10,000 and < 1/1,000), very rare (<1/10,000, including isolated cases). Frequency categories were formed based on clinical studies of the drug and post-registration surveillance.
From the immune system:
rarely - allergic reactions, including hypersensitivity and anaphylactic reactions.
From the nervous system:
Uncommon: paresthesia in the area where the drug is applied.
From the digestive system:
rarely - colitis (including pseudomembranous colitis), hemorrhagic diarrhea, diarrhea, abdominal pain.
For the skin and subcutaneous fat:
very often - burning sensation, erythema, peeling, dry skin in the area where the drug is applied (these phenomena are mostly mild); often - photosensitivity in the area where the drug is applied; infrequently - itching, erythematous rash, dermatitis, worsening acne in the area of application of the drug; rarely - urticaria in the area where the drug is applied.
Other:
rarely - other reactions in the area of application, including hair bleaching.
In studies with topical use of clindamycin as monotherapy, headache and pain in the area of application of the drug were also frequently reported.
Local tolerance
Below are data regarding local tolerability of the drug in patients treated in clinical trials. The severity of local adverse effects was assessed according to the following scale: 0 - none, 1 - weak, 2 - moderate and 3 - severe local adverse effects.
The percentage of patients who had the following symptoms before treatment (baseline) and during treatment was as follows:
Before treatment (baseline) During treatment
Weak Moderate Severe Weak Moderate Severe
Erythema 28% 3% 0 26% 5% 0
Peeling 6% <1% 0 17% 2% 0
Burning 3% <1% 0 5% <1% 0
Dryness 6% <1% 0 15% 1% 0
Contraindications
- hypersensitivity to clindamycin, lincomycin, benzoyl peroxide and/or to any of the components of the drug;
- children under 12 years of age;
- lactation period;
- Crohn's disease, ulcerative colitis, pseudomembranous colitis, incl. in the anamnesis.
Carefully
Concomitant use with other topical acne treatments should be used with caution due to the potential for cumulative skin irritation, which can sometimes be severe, particularly when using products with exfoliating or abrasive properties.
Indoxyl should be used with caution if it is necessary to take it simultaneously with peripherally acting muscle relaxants.
Use during pregnancy and breastfeeding
Pregnancy
There have been no controlled studies in pregnant women using a topical gel containing a combination of clindamycin and benzoyl peroxide.
Data on the use of topical preparations containing one of the active ingredients (clindamycin or benzoyl peroxide) in pregnant women are limited. Preclinical studies do not confirm direct or indirect adverse effects that can be attributed to reproductive toxicity.
Given the low level of systemic exposure to clindamycin and benzoyl peroxide, adverse effects on pregnancy are unlikely. However, the combination of clindamycin and benzoyl peroxide should be used during pregnancy only if the expected benefit to the mother outweighs the potential risk to the fetus.
Lactation period
There is no data on the safety of topical use of the combination of clindamycin and benzoyl peroxide during breastfeeding.
Clindamycin and benzoyl peroxide have poor percutaneous absorption, but there is no data on the excretion of clindamycin or benzoyl peroxide into breast milk when administered topically. Clindamycin is excreted into breast milk after oral and parenteral administration.
The combination of clindamycin and benzoyl peroxide should be used during lactation only if the expected benefit to the mother outweighs the potential risk to the baby.
To avoid accidental entry of the drug into the child’s body when using it during lactation, the drug should not be applied to the skin of the breast.
Fertility
There are no data regarding the reproductive effects of topical clindamycin or benzoyl peroxide.
Use for liver dysfunction
In patients with liver failure, there is no need for dose adjustment.
Use for renal impairment
In patients with renal failure, there is no need for dose adjustment.
Use in children
The use of the drug is contraindicated in children under 12 years of age.
Use in elderly patients
There are no special recommendations for elderly patients.
special instructions
Avoid getting the drug into the mouth, eyes, lips, other mucous membranes, as well as irritated damaged areas of the skin. If the gel accidentally gets into these areas, they should be rinsed well with water.
During the first week of treatment, most patients experience increased peeling and redness of the skin. Depending on the severity of these side effects, patients may use moisturizers, temporarily reduce the frequency of use of the drug, or temporarily discontinue use. The effectiveness of the drug when used less than once a day has not been studied.
If severe skin irritation occurs (eg, severe erythema, severe skin dryness and itching, severe tingling/burning), use of the drug should be discontinued.
Since benzoyl peroxide may cause increased sensitivity to sunlight, you should avoid prolonged exposure to sunlight and do not visit a solarium while using the drug. If sun exposure cannot be avoided, you should use sunscreen cosmetics for your skin and clothing that protects you from the sun's rays.
If the patient has sunburn, you should stop using the drug until the burns are healed.
The drug can discolor hair and colored fabric. Avoid contact of the drug with hair, fabric, furniture or carpeting.
Pseudomembranous colitis
Cases of pseudomembranous colitis have been reported with the use of almost all antibacterial agents, including clindamycin. Their severity varies in severity from mild to life-threatening. Pseudomembranous colitis may occur several weeks after discontinuation of therapy.
Although pseudomembranous colitis is unlikely to occur with topical use of a combination of clindamycin and benzoyl peroxide, if prolonged or severe diarrhea occurs or if the patient suffers from abdominal cramps, treatment should be stopped immediately and a doctor should be consulted, as symptoms may indicate the presence of colitis caused by pseudomembranous colitis. taking an antibiotic.
Resistance to clindamycin
Benzoyl peroxide reduces the likelihood of the emergence of microorganisms resistant to clindamycin. However, patients who have recently used parenteral or topical clindamycin or erythromycin are more likely to have propionobacteria and saprophytic flora with previously acquired antimicrobial resistance.
Cross resistance
The presence of cross-resistance between clindamycin and lincomycin has been established.
Resistance to clindamycin is often associated with resistance to erythromycin.
Impact on the ability to drive vehicles and operate machinery
A negative effect of the drug on the ability to drive vehicles or operate machinery is unlikely.
Overdose
Symptoms:
applying excessive amounts of the drug may cause severe irritation. In such cases, stop using the drug until signs of skin irritation disappear. When applied topically, benzoyl peroxide is primarily absorbed in amounts insufficient to cause systemic effects. If excessive amounts of clindamycin are administered topically, it may be absorbed in amounts that can cause systemic effects. If the combination of clindamycin and benzoyl peroxide is accidentally ingested, adverse reactions from the gastrointestinal tract may occur, similar to the adverse reactions that occur with parenteral use of clindamycin.
Treatment:
Appropriate symptomatic treatment is used to relieve irritation caused by the application of excessive amounts of the drug. Treatment for accidental ingestion should be based on the clinical situation or in accordance with the recommendations of the local National Poison Control Center, if available.
Drug interactions
Because clindamycin exhibits antagonism with erythromycin; Indoxyl should not be used in combination with drugs containing erythromycin.
Clindamycin has been shown to interfere with neuromuscular transmission and, therefore, may enhance the effect of peripherally acting muscle relaxants.
The combination of clindamycin and benzoyl peroxide with tretinoin, isotretinoin and tazarotene should be avoided as benzoyl peroxide may reduce their effectiveness and increase skin irritation. If combination treatment is necessary, the drugs should be administered at different times of the day (for example, one drug in the morning and another drug in the evening).
Using topical products containing benzoyl peroxide concomitantly with topical products containing a sulfonamide may cause a temporary change in the color of the skin and hair at the site of application (yellow or orange).
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature of 2-8°C; do not freeze. Shelf life: 2 years. Do not use after the expiration date stated on the package.
The opened tube should be stored at a temperature not exceeding 25°C for 2 months.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Indoxyl gel (d/nar.50mg+10mg/1g gel tube 25g)
A country
United Kingdom
Country of origin may vary by batch. Please check with the operator for detailed information when confirming your order.
Compound
Tube 25 g Benzoyl peroxide (in terms of anhydrous benzoyl peroxide) 50 mg, clindamycin (in the form of phosphate) 10 mg per 1 g. Excipients: carbomer 2%, dimethicone 0.1%, disodium lauryl sulfosuccinate 0.04%, disodium edetate 0.1%, glycerol 4 %, colloidal silicon dioxide 0.25%, poloxamer 182 0.2%, sodium hydroxide 0.31%, purified water up to 1000 mg. Gel for external use from white to yellowish.
pharmachologic effect
Mechanism of action Clindamycin is an antibiotic of the lincosamide group, which has a bacteriostatic effect against gram-positive aerobic microorganisms and a wide range of anaerobic bacteria. Lincosamides such as clindamycin. bind to the 50S ribosomal subunit of susceptible bacteria and, by interfering with the transport of eptidyl, prevent the elongation of peptide chains. The action of clindamycin is primarily bacteriostatic, however, high concentrations may have a slow bactericidal effect on susceptible strains. Although clindamycin phosphate is inactive in vitro, rapid hydrolysis in vivo converts this compound to active clindamycin. Benzoyl peroxide is a highly lipophilic oxidizing agent with bactericidal and weak keratolytic effect. Benzoyl peroxide has a nonspecific bactericidal mechanism of action, producing reactive oxygen species that prevent the emergence of microorganisms resistant to clindamycin. Pharmacodynamic effects Clindamycin is active in vitro against propionobacterium acnes, which causes acne. There is evidence that P. acnes may develop resistance to clindamycin. Clindamycin inhibits P. acnes in vitro (minimum inhibitory concentration (MIC) 0.4 μg/ml). Once applied, clindamycin reduces free fatty acids on the skin surface from approximately 14% to 2%. Clindamycin reduces inflammation by inhibiting leukocyte chemotaxis. The effectiveness of benzoyl peroxide in the treatment of acne is mainly attributed to its bactericidal activity, especially against P. acnes. The bactericidal activity of benzoyl peroxide is caused by the release of active or free oxygen radicals that can oxidize bacterial proteins. Benzoyl peroxide is effective in the treatment of acne due to its anti-inflammatory and some keratolytic effects. Resistance and cross-resistance Treatment of acne with topical and oral antibiotics such as clindamycin and erythromycin as monotherapy has been associated with the development of antimicrobial resistance in P. acnes as well as in saprophytic flora (eg Staphylococcus aureus. Streptococcus pyogenes). Long-term use of clindamycin may cause the development of resistance in these microorganisms. Benzoyl peroxide is bactericidal and has not caused resistance in P. acnes. The inclusion of benzoyl peroxide in a combination formulation containing 1% clindamycin and 5% benzoyl peroxide reduced the number of clindamycin-resistant P. acnes organisms. The level of acquired resistance for specific organisms may vary depending on geographic location and time. If possible, this should be considered local resistance data, particularly in the treatment of serious infections. Cross-resistance has been established between clindamycin and lincomycin.
Indications for use
Acne (acne vulgaris) of mild to moderate severity, especially with a predominance of inflammatory skin lesions.
Mode of application
Indoxyl is intended for topical use only. The drug should be applied in a thin layer to the entire affected area once a day after thoroughly cleansing the skin with a mild cleanser and completely drying the skin. The gel is poorly absorbed if an excessive amount of the drug is applied to the skin. After applying the gel, you should wash your hands. If necessary, after absorbing the gel, you can use a moisturizer. If dry skin or flaking occurs, you should reduce the frequency of use of the gel or temporarily suspend treatment. The effectiveness of the drug when applied less than once a day has not been studied. Application of the gel more frequently than recommended by the instructions does not increase the effectiveness of treatment, but may increase the risk of skin irritation. The duration of treatment required to achieve a therapeutic effect ranges from 2 to 5 weeks, but not more than 12 weeks. The safety and effectiveness of the combination of clindamycin and benzoyl peroxide in the treatment of acne lasting more than 12 weeks have not been studied. The expected benefit to the patient should be carefully assessed when prescribing treatment for more than 12 weeks of continuous use. Special patient populations Children The safety and effectiveness of the combination of clindamycin and benzoyl peroxide have not been studied in children under 12 years of age, therefore the use of the combination of clindamycin and benzoyl peroxide is not recommended in this population. Elderly Patients There are no specific recommendations for elderly patients. Patients with renal insufficiency No dosage adjustment is necessary. Since the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is weak, renal insufficiency is unlikely to contribute to the occurrence of clinically significant systemic effects. Patients with hepatic insufficiency Dose adjustment is necessary absent. Since the absorption of clindamycin and benzoyl peroxide through the skin when applied topically is weak, the influence of liver failure is unlikely to cause clinically significant systemic effects.
Interaction
Since clindamycin exhibits antagonism with erythromycin, Indoxyl should not be used in combination with drugs containing erythromycin. Clindamycin has been found to interfere with neuromuscular transmission and, therefore, may enhance the effect of peripherally acting muscle relaxants. The combined use of clindamycin and benzoyl peroxide with tretinoin should be avoided , isotretinoin and tazarotene, since benzoyl peroxide can reduce their effectiveness and increase skin irritation. If combination treatment is necessary, the drugs should be used at different times of the day (for example, one drug in the morning and another drug in the evening). Using topical products containing benzoyl peroxide concomitantly with topical products containing a sulfonamide may cause temporary changes in skin color and hair at the site of application of the drug (colored yellow or orange).
Side effect
The adverse events presented below are listed according to the damage to organs and organ systems and the frequency of occurrence. The frequency of occurrence is determined as follows: very often (? 1/10), often (? 1/100 and Contraindications - hypersensitivity to clindamycin, lincomycin, benzoyl peroxide and/or to any of the components of the drug; - children under 12 years of age; - lactation period; - Crohn's disease, ulcerative colitis, pseudomembranous colitis, including a history. With caution Simultaneous use with other local acne treatments should be carried out with caution due to the possible occurrence of cumulative skin irritation, which can sometimes be severe, especially when used agents with an exfoliating or abrasive effect. The drug Indoxyl should be used with caution if it is necessary to take it simultaneously with peripherally acting muscle relaxants. Use during pregnancy and lactation Pregnancy There have been no controlled studies in pregnant women who use a gel for external use containing a combination of clindamycin and benzoyl peroxide was. Data on the use of topical preparations containing one of the active ingredients (clindamycin or benzoyl peroxide) in pregnant women are limited. Preclinical studies do not support direct or indirect adverse effects that could be attributed to reproductive toxicity. Given the low level of systemic exposure to clindamycin and benzoyl peroxide, adverse effects on pregnancy are unlikely. However, the combination of clindamycin and benzoyl peroxide should be prescribed during pregnancy only if the expected benefit to the mother outweighs the potential risk to the fetus. Lactation There is no data on the safety of topical use of the combination of clindamycin and benzoyl peroxide during breastfeeding. Clindamycin and benzoyl peroxide have weak percutaneous absorption, however, there is no data on the excretion of clindamycin or benzoyl peroxide when applied topically into breast milk. Clindamycin is excreted into breast milk when administered orally and parenterally. The combination of clindamycin and benzoyl peroxide should be used during lactation only if the expected benefit to the mother outweighs the potential risk to the child. To avoid accidental exposure of the drug to the child when used during lactation During lactation, the drug should not be applied to the skin of the breast.
Overdose
Symptoms Applying excessive amounts of the drug may cause severe irritation. In such cases, stop using the drug until signs of skin irritation disappear. When applied topically, benzoyl peroxide is mainly absorbed in an amount insufficient to cause systemic effects. If an excessive amount of clindamycin is applied topically, it may be absorbed in an amount that can cause systemic effects. In case of accidental exposure combinations of clindamycin and benzoyl peroxide by mouth may cause adverse reactions from the gastrointestinal tract, similar to adverse reactions that occur with parenteral use of clindamycin. Treatment Appropriate symptomatic treatment is used to relieve irritation caused by the application of excessive amounts of the drug. Treatment in case of accidental ingestion of the drug should be carried out , based on the clinical situation, or in accordance with the recommendations of the local National Poison Control Center, if available.
special instructions
Avoid getting the drug into the mouth, eyes, lips, other mucous membranes, as well as irritated damaged areas of the skin. If the gel accidentally gets into these areas, they should be rinsed well with water. During the first week of treatment, most patients experience increased peeling and redness of the skin. Depending on the severity of these side effects, patients may use moisturizers, temporarily reduce the frequency of use of the drug, or temporarily discontinue use. The effectiveness of the drug when used less than once a day has not been studied. If severe skin irritation occurs (for example, severe erythema, severe skin dryness and itching, severe tingling/burning), use of the drug should be discontinued. Because benzoyl peroxide may cause increased sensitivity to sunlight light, you should avoid prolonged exposure to sunlight, and also not visit the solarium while using the drug. If exposure to the sun cannot be avoided, you should use sunscreen cosmetics for the skin and clothing that protects from sun rays. If the patient has sunburn, you should stop using the drug until the burns are healed. The drug can bleach hair and colored fabric. Avoid contact of the drug with hair, fabric, furniture or carpeting. Pseudomembranous colitis Cases of pseudomembranous colitis have been reported with the use of almost all antibacterial agents, including clindamycin. Their severity varies in severity from mild to life-threatening. Pseudomembranous colitis may occur several weeks after discontinuation of therapy. Although pseudomembranous colitis is unlikely to occur with topical use of the combination of clindamycin and benzoyl peroxide, if prolonged or severe diarrhea occurs or if the patient suffers from abdominal cramps, treatment should be stopped immediately and medical attention should be sought. See your doctor because the symptoms may indicate the presence of colitis caused by taking an antibiotic. Resistance to clindamycin Benzoyl peroxide reduces the likelihood of the emergence of microorganisms resistant to clindamycin. However, patients who have recently used clindamycin or erythromycin for parenteral or topical use are more likely to have propionobacteria and saprophytic flora with previously acquired antimicrobial resistance. Cross-resistance Cross-resistance has been established between clindamycin and lincomycin. Resistance to clindamycin is often associated with resistance to erythromycin. Effect on the ability to drive vehicles and operate machinery. A negative effect of the drug on the ability to drive vehicles or operate machinery is unlikely.