Our doctors
Kolodko Inna Mikhailovna
Doctor - endocrinologist, doctor of the highest category
Experience 26 years
Make an appointment
Slovesnova Tatyana Alekseevna
Endocrinologist, Candidate of Medical Sciences, doctor of the highest category
Experience 48 years
Make an appointment
Mikhailova Elena Vladimirovna
Endocrinologist, diabetologist, candidate of medical sciences
Experience 46 years
Make an appointment
You can undergo a course of treatment for thyrotoxicosis in the endocrinology department of the CELT clinic. We have modern equipment and drugs that allow us to guarantee the desired results.
Features of thyrotoxicosis in children
Hyperthyroidism in childhood is no exception, but the disease is not so common. The cause of the pathology is diseases of the endocrine system, congenital anomalies or lack of treatment of the mother (with female thyrotoxicosis) during pregnancy.
The clinical picture of childhood hyperthyroidism is characterized by the absence of a number of symptoms, first of all, there are no ophthalmological signs. The nature of the disease is benign, and progression can be both rapid and slow.
Symptoms:
- restless night's sleep;
- moodiness, nervousness, emotional instability;
- maintaining elevated temperature;
- tachycardia;
- acceleration in the growth process;
- increased sweating;
- rapid ossification processes;
- sudden “weight loss”;
- deviations in puberty;
- gastrointestinal disorder.
For sick children, hyperthyroidism is dangerous due to metabolic disorders, which lead to severe emaciation and the risk of exhaustion of the body. To prevent the child from becoming exhausted, special attention is paid to nutrition that complies with the recommendations of a nutritionist.
Causes of thyrotoxicosis
The most common cause of thyrotoxicosis is autoimmune damage to the thyroid gland. In this case, a diffuse change in the gland tissue is observed due to the pathological effects of autoantibodies (activating antibodies to the TSH receptor and antibodies to thyroid peroxidase). This type of disorder is characteristic of diffuse toxic goiter and chronic autoimmune thyroiditis.
Another reason why thyrotoxicosis may occur is nodular toxic goiter. This pathology is characterized by the presence of one or more nodular formations that intensively produce hormones and their synthesis is not regulated by the level of TSH.
In addition, the following reasons are distinguished:
- acute or subacute thyroiditis;
- overdose of drugs containing T4 and T3 hormones, taking cordarone;
- excessively large doses of drugs containing iodine;
- the presence of a pathology such as a pituitary tumor that produces the hormone TSH (extremely rare)
- impaired sensitivity of pituitary gland receptors to thyroid hormones.
- transient subclinical thyrotoxicosis in pregnant women (physiological state of the thyroid system in the first trimester of pregnancy).
Types of hyperthyroidism
There are three forms of the disease:
- Subclinical. There are no obvious symptoms, T4 levels are normal, triiodothyronine levels are low;
- Manifest. Characteristic signs of hyperthyroidism appear. T4 level is normal, triiodothyronine level is low;
- Complicated. Symptoms include heart failure, arrhythmia, psychosis and other severe conditions.
According to the level of occurrence of hyperthyroidism, there are:
- primary – pathology of the thyroid gland;
- secondary – the pituitary gland is affected;
- tertiary – processes develop in the hypothalamus.
Clinical manifestations of thyrotoxicosis
Symptoms of thyrotoxicosis are caused by increased basal metabolism under the influence of thyroid hormones, increased breakdown of fats, increased blood glucose levels, destruction of bone and muscle mass, and increased activity of the cardiovascular system.
Clinical manifestations of thyrotoxicosis are as follows:
- sudden weight loss that occurs against the background of normal diet and physical activity;
- excessive sweating;
- rapid heartbeat, which is not associated with cardiovascular diseases;
- increased body temperature, feeling of heat;
- trembling of the whole body, upper and lower extremities;
- difficulty concentrating;
- mood variability;
- stool instability;
- menstrual irregularities in women and a sharp decrease in libido in men;
- muscle weakness;
- enlargement of the eyes (occurs only in diffuse toxic goiter).
The severity of symptoms and their combination depend on the gender and age of the patient, as well as on the concentration of hormones in the blood. With long-term uncompensated thyrotoxicosis, exhaustion of the body as a whole and each organ in particular develops.
A complication of thyrotoxicosis is thyrotoxic crisis. This is an acute condition characterized by severe cardiovascular failure, elevated blood sugar levels, and muscle weakness. The hallmark symptom of thyrotoxic crisis is thyrotoxic psychosis. Clinical signs of a crisis increase very quickly and can be fatal. Timely consultation with a doctor if you suspect thyrotoxicosis allows you to quickly relieve symptoms with the help of special medications and avoid complications.
More about symptoms
The signs of hyperthyroidism listed above are the main ones. Hormone deficiency also causes other symptoms, which depend on the duration of disease progression, the age/gender of the patient and the presence of concomitant diseases.
With thyrotoxicosis, other clinical symptoms are observed:
- increase in neck girth;
- visually noticeable goiter;
- localized swelling in the cervical area;
- feeling of “squeezing” of the larynx;
- absentmindedness;
- instability of emotional state;
- memory problems.
Hyperthyroidism requires mandatory treatment, since the longer the disease develops, the higher the likelihood of serious mental disorders (sharp changes in psycho-emotional state, thoughts of suicide, etc.) that can lead to irreversible mental disorders.
Diagnosis of thyrotoxicosis
Diagnosis of thyrotoxicosis at the CELT clinic begins with an examination by an endocrinologist.
Any diagnosis begins with a survey and examination of the patient. When interviewing the patient, the doctor clarifies the complaints, the timing of their appearance, and the dynamics.
During the examination, the doctor pays attention to visual changes in the thyroid gland, changes during palpation, decreased muscle tone, changes in the cardiovascular system and other organs and systems. Often, one of the first signs of the presence of this pathology is protrusion of the eyes from the orbit of the skull (exophthalmos), which, in turn, leads to a change in the location of the axes of the eyeballs.
After examination, to clarify the nature of thyrotoxicosis and its severity, the doctor prescribes laboratory and instrumental research methods:
- hormonal blood test for TSH, free T4, free T3, antibodies to thyroid peroxidase, TSH receptor
- Ultrasound of the thyroid gland
- radioisotope scan of the thyroid gland
- puncture biopsy of a nodular formation
The course of the disease depending on the gender of the patient
Hormonal diseases are mostly diagnosed in women. This feature is associated with the physiological structure of the female reproductive system, which is highly dependent on hormonal balance.
Symptoms of female thyrotoxicosis can be of varying intensity, depending on the provoking factors. For a woman, hyperthyroidism is a pathology that causes serious problems with the functionality of the internal genital organs and affects appearance.
Due to thyrotoxicosis the following is observed:
- instability of the menstrual cycle, discharge becomes scarcer, pain occurs, and general condition worsens;
- hair loses elasticity, breaks, loses shine and elasticity, and begins to fall out profusely;
- nail plates delaminate and constantly break;
- signs of exophthalmos appear (an increase in the size of the eye opening, the eyeball protrudes);
- the work of the heart muscles accelerates, which leads to tachycardia and arterial hypertension.
The most dangerous is the development of thyrotoxicosis in women during pregnancy. Hormonal endocrine disorder can affect the development of the fetus - treatment during this period is mandatory.
In men
Thyrotoxicosis is diagnosed in men much less frequently, however, it is more severe. Signs of pathological processes in the stronger sex are more pronounced. The main symptom observed in men, regardless of age, is severe muscle weakness. Even athletes lose muscle mass and decrease physical endurance.
Symptoms in men:
- increased irritability with nervousness;
- hyperhidrosis;
- sleep problems;
- hand tremors;
- severe tachycardia;
- increased appetite;
- problems with stool - diarrhea.
Men may also be concerned about impaired potency, which affects the psycho-emotional state and the general condition of the sexual sphere.
Treatment
Specialists from the endocrinology department of the CELT clinic have been successfully treating thyrotoxicosis for several years now.
Based on the examination and diagnostic data obtained, three treatment options are possible:
- conservative treatment, which consists of taking medications that reduce the activity of the thyroid gland;
- surgery involving complete or partial removal of the thyroid gland;
- treatment with radioactive iodine.
Conservative treatment
most often chosen for patients with thyrotoxicosis against the background of diffuse toxic goiter or autoimmune thyroiditis. Treatment, usually long-term, includes taking drugs that block the function of the thyroid gland and the metabolism of hormones in the body (thyreostatics). Treatment is carried out under careful monitoring of general condition, thyroid status (monitoring free T4 every 2 weeks), clinical blood test and biochemical parameters. This is necessary in order to prevent the development of hypothyroidism (low levels of thyroid hormones) and cytotoxic side effects from the drugs. Subsequently, monitoring is carried out somewhat less frequently, once every 2 months, in order to assess the adequacy of the drug dose and maintain persistent euthyroidism (normal levels of thyroid hormones).
Additional medications for the treatment of thyrotoxicosis include beta blockers and prednisolone. They are prescribed in cases of severe thyrotoxicosis and prolonged decompensation.
In case of thyrotoxicosis, therapy with iodine preparations is contraindicated (with the exception of pregnancy, when iodine is necessary for the formation of a normal thyroid gland in the fetus)!
What is hypothyroidism
Hypothyroidism means a deficiency of thyroid hormones in the body, which is manifested not only by its dysfunction, but also by the subsequent failure of the functionality of all organic systems that depend on the action of its hormones. As a result, all metabolic and metabolic biochemical processes in the body slow down, and not only the normal functioning of the nervous, cardiovascular, reproductive and musculoskeletal systems is inhibited, but their anatomical destruction also occurs.
Factors that provoke hypothyroidism can be:
- chronic autoimmune thyroiditis;
- insufficient biosynthesis of thyroid hormones (due to heredity);
- congenital defects in the development of the thyroid gland (hypoplasia or aplasia);
- surgical removal of part of the thyroid gland;
- consequences of exposure of the thyroid gland to radioactive iodine (in the treatment of Graves' disease) or ionizing radiation;
- iodine deficiency in the diet;
- infections and neoplasms of the thyroid gland;
- taking certain medications;
- (in the case of secondary hypothyroidism) pathological conditions of the pituitary gland and hypothalamus.
Hypothyroidism is considered one of the most common endocrine pathologies. According to statistics, this disease most often affects women over 65 years of age, as well as people living in mountainous areas and traditionally experiencing iodine deficiency in their diet.
Introduction
Thyrotoxicosis is a syndrome in which clinical and biochemical manifestations of excess levels of thyroid hormones in the blood are observed, regardless of the reason for the increase in their levels [1].
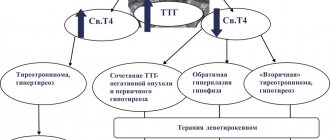
Thyrotoxicosis is reliably indicated by suppression of the level of thyroid-stimulating hormone (TSH) less than 0.1 mU/l (with reference values of 0.4–4.0 mU/l), when detected, additional determination of free fractions of thyroxine (T4) and triiodothyronine (T3) is indicated ) [1]. The first stage of managing a patient with thyrotoxicosis is the differential diagnosis of its causes. The most commonly used classification of thyrotoxicosis syndrome is [1]:
- Thyrotoxicosis caused by increased production of thyroid hormones: diffuse toxic goiter (DTZ);
- multinodular toxic goiter;
- toxic adenoma;
- iodine-induced thyrotoxicosis;
- hyperthyroid phase of autoimmune thyroiditis;
- TSH-related thyrotoxicosis: TSH-producing pituitary adenoma and syndrome of inadequate TSH secretion (resistance of thyrotrophs to thyroid hormones);
- trophoblastic thyrotoxicosis.
- ovarian tumor producing thyroid hormones (Struma ovarii);
- medicinal (overdose of thyroid hormones);
We would like to present an analysis of the medical history of a patient with amiodarone-induced thyrotoxicosis, established only during a thorough examination. This clinical case once again emphasizes the importance of carefully collecting anamnesis at the initial stage of differential diagnosis, as well as clarifying the course of previous cardiovascular diseases in patients with an established excess of thyroid hormones in the blood.
Clinical example
Patient T., 60 years old, came for consultation. At the time of examination (01/10/2019), he complained of heart rhythm disturbances with a tendency to bradycardia, an episodic increase in blood pressure (BP) up to 160/100 mm Hg.
From the anamnesis it is known that rhythm disturbances and increased blood pressure began to be noted in August 2021. I applied for a consultation at the outpatient department of the State Budgetary Institution of Healthcare of the Ministry of Health "Cardiology Center" in Nalchik. Coronary angiography performed in stationary conditions (September 26, 2018) did not reveal significant changes in myocardial blood flow. According to the results of 24-hour Holter monitoring (November 27, 2018), the following disturbances were recorded against the background of sinus rhythm of the heart: single ventricular extrasystole, monomorphic – 19,161 per day (physiological norm up to 700 per day) and paired ventricular monomorphic extrasystole – 17 per day. Against the background of this rhythm with a normal circadian index, the average, maximum and minimum values of heart rate (HR) during the day and night hours are within normal values (128–43). No conduction disturbances or ischemic episodes were detected. The cardiologist prescribed antiarrhythmic and antihypertensive therapy: Allaforte (lappaconitine hydrobromide) 25 mg 2 times a day, Mezinopril (lisinopril) 10 mg 2 times a day, Egilok (metoprolol) 25 mg (1/2 tablet) once a day under blood pressure control and heart rate.
Due to the lack of proper effect from the therapy, to determine further management tactics, the patient was sent for further examination to the Federal Center for Cardiovascular Surgery of the Russian Ministry of Health (Astrakhan), where on November 30, 2018, echocardiography was performed, which revealed hypertrophy of the interventricular septum; regurgitation on the mitral valve of the 1st degree; regurgitation on the tricuspid valve of the 1st degree. Taking into account all the data, the patient was diagnosed with the following: cardiac arrhythmia: frequent monomorphic ventricular extrasystole. Arterial hypertension stage II, risk 3. Chronic heart failure (CHF)-IIA. Ablation of ectopic myocardial foci is recommended.
In January 2021, the patient underwent color duplex scanning of the extracranial sections of the brachiocephalic vessels, which revealed the presence of atherosclerosis of the extracranial sections of the brachiocephalic arteries with stenosis of the carotid bifurcation on the left; non-linearity of the course of the vertebral arteries between the transverse processes of the cervical vertebrae.
To identify possible reasons for the worsening of previous arrhythmias during antiarrhythmic treatment, the patient was examined by an endocrinologist. According to the hormonal profile dated December 12, 2018, hyperthyroidism and T3 toxicosis were revealed: thyroid-stimulating hormone (TSH) <0.001 mU/l (n 0.4–4.0), free T3 – 6.2 pmol/l (n – 2, 6–5.7), free T4 – 21.3 pmol/l (n – 9.0–19.05), antibodies (a/t) to thyroid peroxidase (TPO) – 2.3 U/ml (n <5 ,6). Ultrasound examination (US) did not visualize the thyroid gland. A diagnosis was made: diffuse toxic goiter. Thyreostatic therapy with thiamazole at a dose of 30 mg/day was prescribed.
Diffuse toxic goiter (DTG) is an autoimmune disease that develops as a result of the production of antibodies to TSH receptors (dTSH), clinically manifested by damage to the thyroid gland with the development of thyrotoxicosis syndrome in combination with extrathyroidal pathology [2, 3]. By prevalence among thyroid diseases occurring with hyperthyroidism , DTZ occupies a leading position. Thus, according to the literature, in the world 80–85% of cases of thyrotoxicosis syndrome are caused by thyrotoxicosis [1]. Differential diagnosis is made with neurocirculatory dystonia, thyrotoxic stage of subacute, postpartum or autoimmune thyroiditis, thyrotoxic adenoma, multinodular toxic goiter, amiodarone-induced thyrotoxicosis (AT), follicular thyroid cancer [4].
Additionally, the patient underwent the following studies: blood biochemistry dated January 14, 2019: glucose – 6.63 mmol/l (n – 3.3–6.1), creatinine – 83 µmol/l (n – 72–127), urea – 7.0 mmol/l (n – 1.7–8.3), uric acid – 312 µmol/l (n – 208–428), total bilirubin – 21.7 µmol/l (n – 8.5–20 ,5), alkaline phosphatase – 95 IU/l (n – 30–120), homocysteine – 13.46 µmol/l (n – 5.46–16.20), potassium – 4.29 mmol/l, (n – 3.5–5.1), sodium – 145.7 mmol/l (n – 136–145), chlorides – 108.8 mmol/l (n – 98–112), copper – 15.9 µmol/l (n – 10.99–21.98), magnesium – 0.94 mmol/l (n – 0.73–1.06), zinc – 14.3 µmol/l (n – 11.1–19.5 ), inorganic phosphorus – 0.86 mmol/l (n – 0.81–1.45), selenium – 144.3 μg/l (n – 46–143), ionized calcium – 1.19 mmol/l (n – 1.12–1.32), cholesterol – 3.86 mmol/l, triglycerides – 1.26 mmol/l (n – 0.62–3.51), iron – 22.3 µmol/l (n – 12.5–32.2), folic acid – 4.2 ng/ml (n – 3.1–19.9), vitamin B12 – 216 pg/ml (n – 180–914).
25-OH vitamin D, total (calciferol) as of January 14, 2019: 9.8 ng/ml (n – 30–150), glycosylated hemoglobin – 5.4% (n – less than 6%), tumor markers (CEA) – 2 .95 ng/ml (0–5).
Objectively: height – 178 cm, body weight – 88 kg. Body mass index – 27.8 kg/m2. The condition is of moderate severity. The skin is clean, dark, with moderate moisture. The physique is hypersthenic. Subcutaneous tissue is moderately developed and evenly distributed. Heart rate – 48 per minute. Blood pressure – 130/80 mm Hg. The abdomen is soft and painless. The stool is regular and formed. Diuresis is adequate. The thyroid gland is not palpable, clinically euthyroid. Stable in the Romberg position. Peripheral lymph nodes are not enlarged. At the time of examination there were no signs of adrenal insufficiency.
Despite treatment with thiamazole at a suppressive dose (40 mg/day), laboratory signs of hyperthyroidism persisted. Hormonal profile as of 01/09/2019: TSH<0.0025 mU/l (n – 0.4–4.0), free T3 – 6.6 pmol/l (n – 2.6–5.7), free T4 – 15.91 pmol/l (n – 9.0–19.05), a/t to TPO<3.0 U/ml (n<5.6), parathyroid hormone – 58.8 pg/ml (n – 16.0–87.0), calcitonin <2.0 pg/ml (n<18.2). The level of a/t to rTSH was 1.22 U/l (n<1.0).
The ineffectiveness of the thyreostatic therapy and the questionable result of the study of the level of a/t to rTSH called into question the existing diagnosis of “diffuse toxic goiter”. For further examination and determination of further management tactics, the patient was sent to the Medical Radiological Research Center named after. A.F. Tsyba (Obninsk). Hormonal profile dated January 30, 2019, while taking 40 mg/day of thiamazole without positive dynamics: TSH <0.005 mU/l (n – 0.4–4.0), free T3 – 7.76 pmol/l (n – 3, 1–6.8), free T4 – 23.38 pmol/l (n – 12.0–22.0). The resulting low level of a/t to rTSH of 0.3 U/l (n<1.5) excluded the autoimmune genesis of thyrotoxicosis.
Due to difficulties in visualizing the thyroid gland with ultrasound (see figure), in order to determine its location and size, identify nodes and subsequent differential diagnosis of thyrotoxicosis syndrome, the patient underwent scintigraphy (01/31/2019), the results of which showed the presence of a significantly enlarged thyroid gland, in to a greater extent due to the left lobe with its greater part located behind the bone structures (sternum). The distribution of the radiopharmaceutical (RP) is relatively uniform. No additional foci of drug accumulation were identified in the neck projection. Conclusion: “partially located retrosternally, diffusely enlarged thyroid gland. Multinodular goiter." No clear data were obtained for functional autonomy.
Functional autonomy (FA) of the thyroid gland is an iodine deficiency disease in which persistent pathological hyperproduction of thyroid hormones is caused by the formation of autonomously functioning areas in the thyroid gland. In this case, iodine uptake by thyrocytes and production of thyroxine occurs, independent of the influence of pituitary TSH. The greatest risk of developing thyroid FA is in elderly patients with multinodular goiter, as well as people living in regions with iodine deficiency. A characteristic feature of this disease is the accumulation of radiopharmaceuticals in areas of thyroid hyperfunction during scintigraphy [5, 6].
Based on the results of the examination, the patient was consulted by a radiologist (01/31/2019) and the possibility of radioiodine therapy was proposed after compensation for thyrotoxicosis with a maintenance dose of thiamazole 5–10 mg.
A more detailed history collection revealed that in October 2018, the patient was admitted to the intensive care unit of the cardiology center at his place of residence with arrhythmia, where the antiarrhythmic drug amiodarone was repeatedly administered intravenously. Based on the anamnesis and additional examination results obtained by the endocrinologist, the final diagnosis of “cordarone-induced thyrotoxicosis syndrome, stage of drug subcompensation” was established. Multinodular goiter." Treatment with prednisolone at a dose of 40 mg/day for 2 weeks is recommended against the background of a maintenance dose of thiamazole (5–10 mg/day) and subsequent withdrawal of both drugs under the control of free forms of T4 and T3 in the blood against the background of previously prescribed antiarrhythmic therapy.
Clinically, against the background of therapy and laboratory compensation of thyrotoxicosis (see table), the patient noted an improvement in health: blood pressure and heart rate numbers stabilized. Thus, the patient was diagnosed:
Final: “amiodarone-induced thyrotoxicosis syndrome, stage of drug subcompensation. Multinodular goiter of a partially retrosternally located thyroid gland.” Associated: “heart rhythm disturbance: frequent monomorphic ventricular extrasystole. Arterial hypertension stage II, risk 3. CHF-IIA. Impaired fasting glucose. Osteochondrosis of the cervical spine. Atherosclerosis of the brachiocephalic arteries with stenosis of the carotid bifurcation on the left. Vitamin D deficiency."
Currently, the patient continues to receive previously prescribed antiarrhythmic therapy, as well as a native vitamin D preparation at a dose of 7000 IU/day and remains under dynamic observation with monitoring of the functional state of the thyroid gland once every 6 months.
Discussion
Amiodarone is an iodine-containing drug that has antiarrhythmic and antianginal effects. The drug was developed in 1961 in Belgium for the treatment of angina pectoris, but it turned out to be highly effective in the treatment of life-threatening arrhythmias resistant to other drugs [7]. Due to its pronounced lipophilicity, the drug has a high concentration in tissues and in a number of patients has an effect on thyroid function [8]. The most severe manifestation of amiodarone-induced thyroid dysfunction is thyrotoxicosis. The clinical picture, as a rule, is dominated by cardiovascular disorders: worsening of previous arrhythmias, increased frequency of angina attacks, and the appearance or intensification of signs of heart failure [9–11]. In this regard, patients with cardiac arrhythmias often become patients of an endocrinologist, who, in turn, has to decide on the tactics of treating endocrine disorders in seriously ill patients with cardiac pathology [12].
What is associated with the development of thyrotoxicosis in patients taking amiodarone? The fact is that an amiodarone tablet (200 mg) contains 75 mg of iodine. During metabolism, approximately 6–9 mg of inorganic iodine per day is released from every 200 mg of the drug, which is 50–100 times higher than the daily requirement for the element, which is 150–200 mcg according to WHO [12, 13].
The incidence of thyroid dysfunction ranges from 15 to 20% [14, 15].
Most patients taking amiodarone remain euthyroid. However, some patients may develop hypothyroidism or thyrotoxicosis [16]. The variant of amiodarone-induced thyroid dysfunction depends to a certain extent on the iodine supply of the patient’s region of residence. There is evidence that patients living in areas with high iodine consumption more often develop amiodarone-induced hypothyroidism, and in regions with low iodine consumption - thyrotoxicosis [7]. It should be noted that the place of residence of the patient we presented belongs to regions with moderate iodine deficiency, which increases the risk of him developing hyperthyroidism while taking amiodarone. In addition, the patient had previous changes in the structure of the thyroid gland, such as diffuse nodular goiter.
Thyrotoxicosis can develop both in the first months of treatment and several years after its start. Due to the accumulation of the drug and its metabolite in tissues, as well as slow elimination from the body, the development of AT can begin even several months after discontinuation of the drug [12].
Diagnosis of thyrotoxicosis caused by amiodarone is more difficult than hypothyroidism. Due to the antiadrenergic activity of amiodarone and its blocking effect on the conversion of T4 and T3, the classic symptoms of thyrotoxicosis - goiter, sweating, hand tremors, weight loss - may be mildly expressed or completely absent. The clinical picture is dominated by cardiovascular and mental disorders. With unrecognized and long-term thyrotoxicosis, dilated cardiomyopathy syndrome may develop, manifested by a decrease in the pumping function of the heart and the appearance of heart failure [17].
It is important for the clinician to differentiate the two forms of AT in order to choose the correct tactics for patient management. The first type develops against the background of an initially altered thyroid gland (nodular or diffuse nodular goiter, diffuse toxic goiter, etc.) and is formed according to the type of iodine-induced thyrotoxicosis. Iodine released from the drug leads to an increase in the synthesis of thyroid hormones in the existing zones of autonomy in the gland. It is more common in areas of iodine deficiency. AT type 1 is characterized by normal or increased uptake of 99mTc during scintigraphy. The second type is destructive thyroiditis with destruction of gland cells due to the toxic effect of amiodarone itself, and not just the iodine it contains [18]. Destruction of thyroid cells is accompanied by the release of increased amounts of thyroid hormones into the blood [19, 20]. The main clinical feature of this form is the severity of thyrotoxicosis, incl. development of painful forms clinically similar to subacute thyroiditis. When studying with 99mTc, a decrease in the accumulation of the drug in the thyroid gland was observed. The level of a/t to rTSH does not exceed normal values [21].
For the treatment of AT, thionamides, glucocorticosteroids, plasmapheresis, radioiodine therapy, surgical treatment are used; abroad, potassium perchlorate, a blocker of iodine entry into the thyroid gland, is used [22]. The difference between type 2 AT therapy and type 1 is that treatment is carried out not with large doses of antithyroid drugs (thiamazole 40–80 mg), but with prednisolone at a dose of 40–50 mg/day. The course of treatment can last up to 3 months, because... Cases of resumption of symptoms of thyrotoxicosis have been described when trying to reduce the dose of the drug [21, 23].
In cases where it is impossible to differentiate the two forms of thyrotoxicosis, it is recommended to prescribe 40 mg of thiamazole (or 400 mg of propylthiouracil) and 40 mg of prednisolone and examine the T3 level after 2 weeks. If the level T3sv. decreased by 50% (destructive thyrotoxicosis), thiamazole should be discontinued and prednisolone should be continued if T3. decreased by less than 50% (increased synthesis of thyroid hormones) or did not change - discontinue prednisolone, continue taking thiamazole (or propylthiouracil) [21].
Surgical treatment is usually carried out if it is impossible to achieve compensation for the disease after a long (about 6 months) course of drug therapy or when AT is combined with nodular toxic goiter. In areas with borderline iodine deficiency, patients with diffuse or nodular goiter with normal or increased uptake of the radioisotope, in the absence of effect from conservative therapy, are indicated for treatment with radioactive iodine.
Conclusion
In the presented clinical case, amiodarone-induced thyrotoxicosis syndrome, which is quite rare in clinical practice, occurs. Difficulties in the primary diagnosis of amiodarone-induced thyrotoxicosis syndrome in the patient presented in the article were due to a number of reasons. First, there is no history of amiodarone use. Only a targeted search for drug causes of thyroid hyperfunction made it possible to clarify important anamnestic data. Secondly, there are difficulties with palpation and ultrasound visualization of the thyroid gland due to its retrosternal location. Thirdly, in the region of residence there is no possibility of carrying out scintigraphy, a highly informative research method that allows differential diagnosis of many causes of thyrotoxicosis syndrome with great accuracy. Features of the management tactics of the presented patient include therapy with glucocorticosteroids while continuing to take thiamazole.
For successful management of a patient with thyrotoxicosis syndrome and a good outcome of the disease, it is important to be able to promptly identify symptoms of thyroid hyperfunction, carry out differential diagnosis of its possible causes, assess the characteristics of the course of the disease, and prescribe adequate pathogenetic and symptomatic treatment. For elderly patients, it is the cardiac effects of excess thyroid hormones that pose a serious danger, such as worsening the course of concomitant forms of arrhythmias, increased frequency of angina attacks and episodes of syncope. Worsening of existing arrhythmias in patients taking amiodarone is an indication for examining the functional state of the thyroid gland.
Patient consent
Patient T., presented in the clinical case, voluntarily gave verbal consent to the publication of personal medical information. There is no written consent due to the remoteness of the patient’s residence from the medical center.
Severity
Depending on how serious the poisoning of the body by thyroid hormones is, there are three degrees of severity:
Severe degree . Occurs in cases where thyrotoxicosis has already been observed previously, but has not been cured. If we talk about the dangers of thyrotoxicosis in severe form, we can immediately note that disturbances appear in the functioning of many internal organs. Even vision can be affected - the tissues of the orbit of the eye swell and they protrude. This makes it difficult to focus your vision.
Average degree . Typically, it is at this stage that the heart rate begins to increase and weight begins to decrease. In this case, problems with digestion and adrenal glands are observed.
Mild degree . This is a form of thyrotoxicosis that is difficult to detect - only the thyroid gland is affected and the heart rate is slightly increased - all other body systems are normal and do not suffer from hormonal imbalance.