Verrukacid solution for external use fl 2g
Active substance
phenol
ATX code
D11AF (Drugs for the treatment of calluses and warts)
Release form, packaging and composition of the drug
◊ Solution for external use
in the form of a mobile oily liquid from pinkish or light brown to brown with the smell of phenol.
| 1 g | |
| phenol | 588 mg |
| metacresol | 392 mg |
[PRING] ethanol 95% 10 mg, purified water up to 1 g.
2 g - dark glass bottles (1) complete with or without applicators - cardboard packs. 10 g - dark glass bottles (1) complete with or without applicators - cardboard packs.
Clinical and pharmacological group
A drug with cauterizing and mummifying effects
Pharmacotherapeutic group
Local necrotizing agent
Indications for use
- common, filiform and plantar warts;
- papillomas;
- genital warts of the skin;
- dry calluses;
- keratomas.
Dosage
Verrucacid is for external use only.
The drug is applied precisely to the treated area with a special applicator or a small thin wooden stick, preventing it from coming into contact with adjacent healthy areas of skin and mucous membranes.
For small papillomas (up to 2 mm in size) and filiform warts, Verrucacid is applied once.
Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrucacid is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrucacid 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Contraindications
- increased individual sensitivity to the components of the drug;
- pigmented nevi (moles);
- rashes located on the red border of the lips and mucous membranes;
- Do not apply the drug to the surface of the skin with an area of more than 20 cm2;
- children's age up to 7 years.
Overdose
Not identified.
Side effects
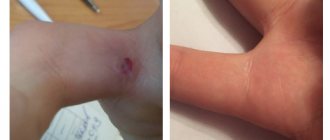
Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents. Swelling and redness of the skin when applied to the eye area, which goes away on its own. Allergic reactions.
Overdose
The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid with any ointments.
Storage conditions
In a place protected from light and out of reach of children at a temperature of 18 to 22°C. Shelf life: 5 years.
Conditions for dispensing from pharmacies
Over the counter.
Special Instructions
Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; Do not reapply the drug before the dates indicated above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Verrukacid 2g solution for external use
pharmachologic effect
It has a cauterizing effect and coagulates skin proteins.
Composition and release form Verrukacid 2g solution for external use
Solution - 1 g:
- Active ingredients: phenol 588 mg, metacresol 392 mg.
- Excipients: ethanol 95% 10 mg, purified water up to 1 g.
2 g - dark glass bottles (1) complete with or without applicators - cardboard packs.
Description of the dosage form
A solution for external use in the form of a mobile oily liquid from pinkish or light brown to brown with a phenol odor.
Directions for use and doses
Verrucacid is for external use only.
The drug is applied precisely to the treated area with a special applicator or a small thin wooden stick, preventing it from coming into contact with adjacent healthy areas of skin and mucous membranes.
For small papillomas (up to 2 mm in size) and filiform warts, Verrucacid is applied once.
Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrucacid is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrucacid 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Indications for use Verrukacid 2g solution for external use
- Common, filiform and plantar warts;
- papillomas;
- genital warts of the skin;
- dry calluses;
- keratomas.
Contraindications
- Increased individual sensitivity to the components of the drug;
- pigmented nevi (moles);
- rashes located on the red border of the lips and mucous membranes;
- Do not apply the drug to the surface of the skin with an area of more than 20 cm2;
- children's age up to 7 years.
Application of Verrukacid 2g solution for external use during pregnancy and breastfeeding
The use of the drug is possible in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child. During lactation, it is not recommended to remove formations located on the mammary glands and hands.
Use in children
Contraindicated for children under 7 years of age.
special instructions
Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; Do not reapply the drug before the dates indicated above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Overdose
Not identified.
Side effects Verrukacid 2g solution for external use
Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents. Swelling and redness of the skin when applied to the eye area, which goes away on its own. Allergic reactions.
Drug interactions
The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid with any ointments.
Verrucacid solution for external use approx 2 g fl/pack of cards x1
Trade name: Verrukacid®. INN or group name: phenol, metacresol. Dosage form: solution for external use.
Compound:
Active ingredients: phenol – 588 mg, 3-methylphenol (metacresol) – 392 mg. Excipients: ethyl alcohol (ethanol) 95% - 10 mg, purified water up to 1 g.
Description: mobile oily liquid from pinkish or light yellow to brown with the smell of phenol. Pharmacotherapeutic group: local necrotizing agent. ATX code (D11AF).
Pharmacological properties Has a cauterizing effect, coagulates skin proteins.
Indications for use: Common, filiform and plantar warts, papillomas, genital warts of the skin, dry calluses, keratomas.
Contraindications Increased individual sensitivity to the components of the drug. Pigmented nevi (moles), rashes located on the red border of the lips and mucous membranes. Do not apply the drug to the skin surface area of more than 20 cm2. Children under 7 years old.
Pregnancy and breastfeeding The use of the drug is possible in cases where the expected benefit to the mother outweighs the potential risk to the fetus or child. During lactation, it is not recommended to remove formations located on the mammary glands and hands.
Directions for use and dosage Verrukacid® is intended for external use only.
The drug is applied precisely to the treated area with a special applicator or a small thin wooden stick, preventing it from coming into contact with adjacent healthy areas of skin and mucous membranes. For small papillomas (up to 2 mm in size) and filamentous warts, Verrukacid® is applied once. Larger papillomas and small warts (2-3 mm in size) are smeared with the drug 3-4 times, taking breaks to allow the applied liquid to dry.
Before removing warts with a dense keratinized surface on the hands, plantar warts, keratomas, and dry calluses with Verrucacid®, it is necessary to remove horny layers from their surface. To do this, apply a keratolytic ointment (for example, 10% salicylic or other) for several hours, covering the lubricated area with compress paper or plastic film, and then use a gauze bandage. Possible sealing with adhesive tape. After this, the bandage or adhesive plaster is removed, the skin is steamed in hot water with the addition of soap and soda for 10-15 minutes and the horny layers are removed (cut off with nail scissors or tongs). Verrukacid® is applied to dried skin several times, taking 3-4 minute breaks to allow the drug to dry.
Warts on the hands and soles are treated with Verrukacid® 7-10 times with an interval of 3-4 minutes.
It is enough to treat keratomas and dry calluses 3-4 times with an interval of 3-4 minutes.
If it is necessary to apply the drug repeatedly, in order to avoid burns to the surrounding skin, it is advisable to lubricate it with zinc paste. The paste is removed with a dry gauze swab after the last portion of Verrukacid® has dried.
It is not recommended to remove genital warts yourself; they are treated with Verrucacid® in the treatment room of the clinic by a dermatovenereologist or urologist. The drug Verrukacid® is applied to each element separately 1-2 times with an interval of 3-4 minutes.
Repeated treatment, if necessary, is carried out 6-8 days after the crust falls off. 4-5 procedures are allowed.
Side effects Burn (in case of contact with healthy skin). If the drug accidentally comes into contact with healthy skin, it is necessary to immediately, carefully, without rubbing, remove it from the skin, and then treat the affected areas with 10-40% ethyl alcohol or alcohol-containing liquids (vodka, lotion, cologne) and wash thoroughly with warm water and soap. If a burn occurs, it is recommended to use anti-burn and healing agents.
Swelling and redness of the skin when applied to the eye area, which goes away on its own.
Allergic reactions.
Overdose Not detected.
Interaction with other drugs The components of the drug easily dissolve in the ointment base, and therefore it is not recommended to lubricate the skin area treated with Verrucacid® with any ointments.
Special instructions Do not allow the drug to come into contact with mucous membranes, especially the mucous membrane of the eyes. In case of contact, immediately rinse eyes with plenty of water and consult an ophthalmologist.
It is unacceptable to bandage the areas treated with the drug, seal them with a plaster, lubricate them with any ointments and remove the crust; you cannot reapply the drug before the time limits specified above. Clothing made of synthetic fabrics should not touch the area of skin treated with the drug.
It is not recommended to apply Verrucacid® to formations located in skin folds (inguinal folds, anal area, interdigital spaces, etc.) and heavily sweating areas in order to avoid burns to unaffected skin of the contacting surface or as a result of the drug spreading over wet skin.
The area of skin treated with Verrucacid® must be air-dried; it must not be lubricated with any ointments or washed with water on the first day after treatment.
When used correctly, the drug does not leave scars.
Particular care and caution is required when treating children with the drug.
Release form Solution for external use in dark glass bottles with and without applicator, 2 and 10 g.
Each bottle, along with instructions for medical use, is placed in a cardboard pack.
Additionally, each cardboard pack can include a plastic lid with an applicator.
Storage conditions In a place protected from light at a temperature of 15 to 25 ° C.
Keep out of the reach of children.
Shelf life: 5 years. Do not use after expiration date.
Conditions for dispensing from pharmacies Dispense without a prescription.