Elocom®
When applied to large areas of skin for a long time, especially when using occlusive dressings, systemic action of GCS may develop. Given this, patients should be monitored for signs of suppression of the function of the hypothalamic-pituitary-adrenal system and the development of Itsenko-Cushing syndrome.
Avoid contact of Elocom® with the eyes.
Propylene glycol stearate, which is part of the drug, may cause irritation at the site of application. In such cases, use of the drug should be discontinued and appropriate treatment should be prescribed.
It should be borne in mind that GCS can change the manifestations of some skin diseases, which can complicate the diagnosis. In addition, the use of GCS may cause a delay in wound healing.
With long-term therapy with GCS, sudden cessation of therapy can lead to the development of rebound syndrome, manifested in the form of dermatitis with intense redness of the skin and a burning sensation. Therefore, after a long course of treatment, the drug should be discontinued gradually, for example, by switching to an intermittent treatment regimen before stopping it completely.
With systemic and local (including intranasal, inhalation and intraocular) use of GCS, visual impairment may occur. If a patient has symptoms such as blurred vision or other visual disturbances, the patient should be advised to consult an ophthalmologist to identify possible causes of visual disturbances, including cataracts, glaucoma or rare diseases such as central serous chorioretinopathy (CSC), which have been observed in a number of cases with systemic and local use of GCS.
Any of the side effects described with systemic use of glucocorticosteroids, including suppression of adrenal function, may also occur with topical use, especially in children.
When treating psoriasis, GCS should be used with caution, since in some cases, relapses of the disease, the development of tolerance to the drug, the risk of generalized pustular psoriasis and the development of local or systemic toxic reactions due to impaired skin barrier function have been reported. If a secondary infection occurs, appropriate antibacterial therapy should be carried out. If there are any signs of infection spreading, it is necessary to stop the external use of GCS and carry out appropriate treatment with antibacterial or antifungal drugs. If there are signs of hypersensitivity or skin irritation associated with the use of the drug, you should stop treatment and consult a doctor. On the skin of the face more often than on other surfaces of the body, after long-term treatment with topical corticosteroids, atrophic changes may appear; the course of treatment in this case should not exceed 5 days. Use with caution in persons with existing atrophic skin changes, especially in the elderly. Care must be taken when applying the drug to the face, folds, natural folds, and areas with thin skin.
Use in pediatrics
Due to the fact that in children the ratio of surface area to body weight is greater than in adults, children are at greater risk of suppressing the function of the hypothalamic-pituitary-adrenal system and developing Cushing's syndrome when using any topical corticosteroids. Long-term treatment of children with GCS can lead to disturbances in their growth and development.
Children should receive the minimum dose of the drug sufficient to achieve the effect.
Long-term use of the drug in children should be carried out under the supervision of a physician. In children, the course of treatment should not exceed 5 days. Occlusive dressings should not be used.
Elokom in complex therapy of chronic dermatoses
P
The problem of treating chronic dermatoses is currently acquiring special scientific, practical and social significance due to the increase in the number of patients, their social maladaptation, and the resistance of certain clinical forms to therapy [2]. The emergence of corticosteroids, cytostatics, antibiotics, and effective new generation antihistamines has significantly expanded the possibilities of rehabilitation of patients and changed the prognosis for many skin diseases. However, it is difficult to imagine treating patients with severe, torpid forms of dermatoses without the use of external medications that include corticosteroid hormones.
Local glucocorticosteroids (GCS)
have been used in medical practice for more than 40 years; over time, pharmacologists have changed their structure to achieve higher effectiveness of external agents. Thus, the inclusion of a butyric acid residue in the structure of hydrocortisone leads to the formation of hydrocortisone butyrate with more pronounced anti-inflammatory properties. Modification of the GCS molecule by introducing halogens (chlorine, fluorine) increased their effectiveness even more significantly [6,15,16]. The therapeutic effect of external corticosteroids is due to their anti-inflammatory, vasoconstrictor, antiallergic and antiproliferative effects [7,13,14]. The anti-inflammatory effect of local GCS in the skin and other tissues is realized through various mechanisms, but the mechanism mediated by cytosolic receptors is of greatest importance [1,7,8]. Its essence is that the hormone-receptor complex, penetrating into the nucleus of target cells in the skin (keratinocytes, fibroblasts, neutrophils, eosinophils, lymphocytes), increases the expression of genes encoding the synthesis of peptides called lipocortins. They inhibit the activity of lysosomal phospholipase A2 and thereby reduce the formation of inflammatory mediators - eicosanoids (prostaglandins, leukotrienes) from phospholipids. In addition, acting on granulocytes and lymphocytes, corticosteroids inhibit their activity [12]. The mechanism of the vasoconstrictive effect of corticosteroids has not been sufficiently studied: it is probably due to the narrowing of metarterioles. The antiproliferative effect of GCS is associated with inhibition of the synthesis of nucleic acids, primarily DNA, in the cells of the basal layer of the epidermis and fibroblasts of the dermis [5,10,11].
Thus, with the local use of steroid-containing ointments, it seems possible to achieve the main therapeutic goal - relieving itching and reducing inflammation in the skin.
The generally accepted classification of local corticosteroids is based on their chemical structure and activity. According to the severity of the therapeutic effect, they are assessed as weak, moderate, strong and very strong (Table 1) [15]. Depending on the chemical structure, local corticosteroids are divided into fluorinated, doubly fluorinated and non-fluorinated. Non-fluorinated corticosteroids, compared to fluorinated ones, are usually less effective, but safer with regard to the development of adverse reactions during therapy. In this regard, the synthesis of a drug comparable in potency to fluorinated corticosteroids, but with less pronounced side effects, is extremely relevant [3,5].
This problem was solved with the appearance on the pharmaceutical market of a new non-fluorinated corticosteroid,
Elokom
. Elokom is a topical GCS with a new, unique molecular structure. Replacing elements in the 9th and 21st positions with chlorine in its chemical structure allows Elokom to combine high activity with the absence of side effects characteristic of fluorine-containing drugs. Compared to other non-fluorinated corticosteroids, it has high therapeutic efficacy and, according to the results of clinical trials, is classified as a potent drug [6,15,18].
As a result of numerous studies aimed at finding an effective and safe steroid drug for external therapy, the possibility of enhancing the effect of the steroid not only by halogenation, but also by esterification (formation of esters) was determined. Steroid esters have low affinity for receptors, but the presence of esterases in the skin makes it possible to cleave ester bonds and leads to activation of the steroid at the site of action [7,8].
Elokom contains a synthetic 17-heterocyclic corticosteroid - mometasone furoate. The inclusion of a furoate ring in the corticosteroid molecule gives Elokom a number of positive qualities, providing a long-lasting effect after application (within 25–30 hours). This allows you to use it once a day.
One more important property of Elokom should be highlighted: thanks to its structural modification, the drug maximally (compared to other corticosteroids) inhibits the synthesis of pro-inflammatory cytokines (IL-1, IL-6, TNF-alpha). Thus, the use of Elokom should be regarded as pathogenetically justified, since pro-inflammatory cytokines are the key mediators of inflammation in the skin (primarily in allergic dermatoses and psoriasis).
The distinctive features of Elokom include high security
, since it does not cause skin atrophy, hypertrichosis, folliculitis - side effects characteristic of fluoride-containing drugs. In addition, the penetration of the drug and its metabolites from the skin into the blood is insignificant, the half-life is short, and binding to transcortin is high, which determines the virtual absence of systemic side effects (the drug does not inhibit the hypothalamic-pituitary-adrenal system).
Recently, data have appeared indicating that Elokom has an extra-genomic effect, i.e. ability to modulate inflammation without interacting with transcription factors. Apparently, this property largely determines the local and systemic security of Elokom (Emelyanov A.V., Monakhov K.N.).
An important advantage of the drug can be considered its existence in three dosage forms (0.1% cream, 0.1% ointment, 0.1% lotion), which determines the possibility of its use at different stages of the inflammatory process with different localization. All three dosage forms
have a good base that maintains skin pH. The ointment promotes optimal penetration of the drug into the skin and does not contain allergens. The cream, thanks to its composition, created as an oil in water, concentrating in the upper layers of the epidermis, allows you to gently stop acute inflammatory processes. The base of the cream makes it possible to use it instead of previously used lotions. The fat-free base of the lotion ensures its easy distribution over the surface of the skin without sticking or drying out the hair, it does not leave visible marks and has a cooling effect on the skin.
Contraindications for prescribing Elokom coincide with the contraindications for most topical corticosteroids. These include individual intolerance to the drug, viral and bacterial skin infections, rosacea. The drug is not recommended during pregnancy and breastfeeding.
When studying the therapeutic activity of Elokom, 119 patients were under our supervision
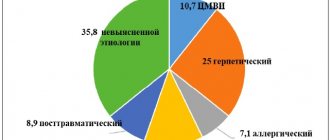
aged from 12 to 70 years (66 women and 53 men). According to nosological forms, patients were divided into groups: group 1 – psoriasis (n=33), group 2 – atopic dermatitis (n=32), group 3 – eczema (n=26), 4 – dermatitis (n=19); 5 – other dermatoses (discoid lupus erythematosus, lichen planus, n=9).
63% of patients (n=75) had concomitant pathologies: chronic rhinitis, pharyngitis, gastritis, cholecystitis, bronchial asthma, in women - mastopathy, intestinal dysbiosis, cervical erosion, neurocirculatory dystonia, spinal osteochondrosis.
Among the supposed factors that caused an exacerbation of the pathological process on the skin, we can note the impact of irritating factors directly on the skin, stressful situations, poor diet, abortion, and pregnancy.
All patients had previously received desensitizing, anti-inflammatory enzyme preparations and other symptomatic treatment. A history of use of corticosteroid ointments was indicated by 95% of patients (n=113).
Elokom was included in traditional complex treatment or prescribed as monotherapy.
The choice of dosage form depended on the clinical symptoms of the disease (the severity and localization of the skin process, the severity of skin infiltration, the presence of oozing, etc.). The ointment was applied in a thin layer, lightly rubbing, onto the affected areas of the skin of the torso and limbs in places with severe infiltration, lichenification in the chronic course of the process, the cream was applied to the face and neck in acute or subacute inflammation with pronounced symptoms of weeping and erythema. The lotion was applied to the scalp.
Depending on the achieved clinical effect, treatment was continued from 14 to 28 days.
The obtained clinical results of treatment of patients were assessed according to the following criteria: clinical recovery - complete resolution of the rash; significant improvement – reduction in the number of rashes by 60–80%; improvement – reduction of rashes by 50% or less.
Elokom in the treatment of allergic dermatoses
Among patients with atopic dermatitis
There were 14 men and 18 women, and 19 of them suffered from atopic dermatitis from 1 year of age. In 10 patients, pathological skin changes were located in the elbow and popliteal folds, in 9 – on the skin of the distal parts of the upper extremities; 18 had rashes around the mouth. In 7 patients, the pathological process diffusely involved the skin of the face, the back and side of the neck, and the upper part of the chest. In 17 patients the process was acute, in 15 it was subacute. Erythema, small-papular elements, pronounced lichenification, numerous excoriations and peeling were detected on the affected skin areas. The rashes occupied from 6 to 24% of the total body surface (when determining the area of affected skin, we used the palm rule, used in examining patients with thermal burns [4]. Patients were bothered by itching.
In the group of patients with atopic dermatitis, a decrease in hyperemia, peeling, and itching was noted on days 1–2 of treatment with Elokom ointment or cream. On days 10–12, these phenomena resolved almost completely in 75% of patients (n=24), i.e. clinical recovery was observed. Significant improvement was noted in 16% of patients (n=5). Long-term foci of infiltration regressed more slowly: in 9% of patients (n=3) by 20–24 days of treatment they remained in the area of the elbow and knee bends.
Group of patients with chronic eczema
included 26 people (12 men and 14 women) with disease duration from 1 to 20 years. The pathological skin process was represented by rashes that were localized mainly on the palms and soles, where lesions with dryness, infiltration, scales, crusts, and painful cracks were noted; only in 8 patients the process was widespread.
Patients with eczema who received monotherapy with Elok cream noted improvement on days 2–4 of treatment. On days 8–12, we observed clinical remission in 58% of patients in this group, and significant improvement in 42%. In patients with widespread eczema, 2–3 days after the start of complex treatment using Elokom, a decrease in hyperemia, peeling, swelling, and itching was observed. By 12–14 days of treatment, resolution of the skin process in this group occurred in 90% of patients (n=7); In 1 patient, positive trends were observed in the resolution of skin efflorescence.
In 12 patients with dermatitis
(n=19, 9 men, 10 women) the skin process was characterized by limited acute erythema of various localizations with symptoms of weak vesiculation and weeping. In 7 patients, seborrheic dermatitis with localization of the process on the scalp was observed.
We noted the high therapeutic effectiveness of Elokom cream in patients with simple contact dermatitis, when 2-3 applications of the drug were sufficient to resolve the process. In allergic contact dermatitis, complete recovery was noted on days 3–5 of treatment.
Elokom in the treatment of psoriasis
The group of patients with psoriasis (n=33) consisted of 18 men and 15 women. The duration of the disease ranged from 2 months to 20 years. 27 of these patients were diagnosed with vulgar psoriasis (20 cases – progressive stage, 7 – stationary stage); in 4 – exudative, in 2 – erythroderma. In 14 patients the skin process was limited, in 19 it was widespread. The rashes were represented by typical psoriatic papules and plaques with severe and moderate infiltration, with silver-white scales on the surface and a positive psoriatic triad. In patients with a widespread process, the disease was accompanied by disseminated rashes located on the skin of the torso, upper and lower extremities, and on the scalp; single plaques were localized on the skin of the neck and face. Subjectively, 90% of patients noted skin itching of varying degrees of intensity.
Patients with limited process (n=14) were prescribed monotherapy with Elokom ointment. 19 patients with widespread psoriasis received complex therapy, including antihistamines, non-steroidal anti-inflammatory drugs, vitamins, hepatoprotectors and external Elokom ointment.
Under the influence of therapy with Elok ointment in patients with limited psoriasis, a decrease in the intensity of the color of papules, peeling and a decrease in itching was noted on days 5–6 of treatment, and by days 16–18 the process regressed in 43% (n=6) of patients in this group, leaving depigmentation. 35% of patients (n=5) noted a significant improvement in skin status, 22% (n=3) - improvement. In the group with an advanced process who received complex therapy, we observed a clinical effect of varying severity. Improvement was noted 8–10 days after the start of treatment in 60% of patients (n=12), 40% (n=7) showed a positive trend in the course of dermatosis. Further therapy led to a significant regression of psoriatic lesions and their resolution in 79% of patients (n=15) by days 20–22 of treatment. In 4 patients, there was a decrease in itching and peeling with persistent infiltration of the lesions, i.e. 21% of patients showed improvement as a result of Elocom therapy.
Elokom lotion was used by patients with lesions of the scalp (n=9). Significant improvement was noted on days 4–6 of treatment. Almost complete resolution of psoriatic plaques on the scalp occurred in all patients within 14–15 days from the start of treatment.
It seems interesting to include Elokom in the regimen of staged corticosteroid therapy for psoriasis. In particular, a number of authors recommend starting external therapy for vulgar psoriasis with combination preparations containing glucocorticosteroid and salicylic acid (Diprosalic ointment/lotion). Salicylic acid ensures optimal penetration of the corticosteroid into the site of inflammation, normalizing the processes of keratinization. The average duration of use of Diprosalik is 2–3 weeks. In the future, a transition to Elokom (ointment/lotion) is indicated - an active inhibitor of pro-inflammatory cytokines, which is used until complete regression of the rash. This scheme makes it possible to increase the effectiveness of external therapy for psoriasis (Arabiiskaya E.R., Sokolovsky E.V.).
Elokom in the treatment of discoid lupus erythematosus, lichen planus
In this group of patients, complete resolution of the pathological skin process could not be achieved, however, an improvement in skin status was observed in 80% of patients (n=7). Thus, in 2 patients with discoid lupus erythematosus (disease duration – 5 and 7 years), in whom the skin process was characterized by a persistent, constantly relapsing course, pronounced hyperkeratotic changes, we noted positive dynamics on days 5–8. Skin infiltration decreased, scales disappeared, erythema resolved on days 20–22, and hyperkeratosis decreased. External use of Elokom was combined with general therapy (delagil, angioprotectors, antihistamines, calcium supplements). In patients with lichen planus (n=7), there was a slight slowdown in the resolution of papules, which continued to persist until 18–22 days from the start of therapy in 4 patients; In three cases the rash resolved completely.
The time frame for resolution of the main clinical symptoms of dermatoses during treatment with Elocom is shown in Table. 2.
During treatment with Elocom, in 2 patients we noted adverse reactions in the form of a sharp increase in itching and hyperemia after the first application of the drug, which is why it was discontinued. Laboratory data (clinical blood test, general urine test, biochemical blood test) during treatment remained within normal limits, which indicates the high safety of the drug. Noteworthy is the fact that even after 4 weeks of using Elokom cream or ointment, no side effects were identified.
Thus, clinical studies show that Elokom, which has antipruritic, anti-inflammatory, vasoconstrictive and antiproliferative effects, is the drug of choice in the treatment of chronic dermatoses.
The rapid onset of a positive clinical effect, ease of use due to universal dosage forms, cost-effectiveness, almost complete absence of side effects when used correctly, safety, the newest basis of the drug, excluding systemic effects, reduction of treatment time indicate that the drug has opened up new prospects in the treatment of skin diseases. diseases.
Literature:
1. Babayants R. S., Konstantinov Anti-inflammatory ointments in dermatological practice, Medicine, 1974.
2. Grebenyuk V.N., Toropova N.P., Kulagin V.I. Russian Congress of Dermatologists and Venereologists, Kazan, 1996, pp. 4-5
3. Danilov V. N., Piryatinskaya V. A, Lalaeva A. M. Elocom - effectiveness and safety in dermatological practice, Bulletin of Dermatology and Venereology, 1998, N5, p. 53-55
4. Murazyan R. I, Panchenkov N. R. Emergency care for burns, M., Medicine, 1983
5. Samgin M. A., Sevidova L. Yu. Efficacy of laticort in steroid-sensitive dermatoses Russian Journal of Skin and Venereal Diseases, 1998, N1, 37-38
6. Shakhtmeister I. Ya., Shvarts G. Ya. New drugs in dermatology, Moscow, 1996.
7. Shakhtmeister I. Ya., Shimanovsky N. L. Problems of improving pharmacotherapy of inflammatory and allergic dermatoses using external drugs of glucocorticoid nature. Bulletin of Deratology and Venereology, 1998, N2, p. 27-30
8. Shakhtmeister I. Ya., Shimanovsky N.L. New opportunities in the treatment of inflammatory and allergic dermatoses using an external glucocorticosteroid drug - advantan, Bulletin of Dermatology and Venereology, 1999, N2, p. 51-53
9. Barton BE, Jakway JP, Smith SRCytokine inhibition by a Novel Steroid, Mometasone Furoat, Immunpharmacol. and Immunotoxicol., 1991, 3, 251-261
10. Catz HJ, Prawer SE, Watson MJ et al Mometasone furoat ointment 0.1% in psoriasis, J. Dermat., 1989, 28, 342-345
11. Cornell RC Correlation of the vasoconstriction assay and clinical activiti in psoriasis, Arch. dermatol., 1985, 121, 63-66
12. Groth O, Juhlin L, Michaelson G et al, Acta Derma. Venerol.,1967,vol.47,p.216-221
13. Medansky RS, Brody NJ Kanof NB et al. Semin. Dermstol. 1987, vol. 6 p.m. 94-100
14. Medansky RS, Brody NJ Kanof NB et al. Semin. Dermstol. 1987, vol. 6 p.m. 94-100
15.Miller JA, Munro DD Drugs 1980, 119-134
16. Popper TL, Gentles MJ Kung TT et al. Structure-activity relation-ship of series of novel topical corticosteroids
17. J Steroids Biochem 1987; 27:837-842
18. Rosenthal D. Duke EA A Clinical investigation of the efficacy and safety of mometasone furoate ointment 0.1% vs betamethasone valerate ointment 0.1% in treatment of psoriasis Curr Ther Res 1988; 5: 790-793.
19. Swinehart JM, Barkoff JR Dvorkin D. et al. Mometasone furoate lotion once daily versus triamcinolone acetonid lotion twice daily in psoriasis. Int. J. Dermatol. 1989; 28:680-683
20. Vernon HJ Lane AT Waston W. Comparison of mometasone furoate 0.1% cream in the treatment of children atopic dermatitis J.Am. Acad. Dermat. 1991; 24; 603-606.
Elokom
Use during pregnancy and breastfeeding
The safety of mometasone furoate during pregnancy and lactation has not been studied.
GCS penetrate the placental barrier. Long-term treatment and use in high doses during pregnancy should be avoided due to the risk of negative effects on fetal development.
GCS are excreted in breast milk. If it is necessary to use GCS in high doses and/or for a long time, breastfeeding should be stopped.
Use in children
Contraindication: children under 2 years of age (due to insufficient data).
Caution should be exercised when applying the drug to the face and intertriginous skin, using occlusive dressings, and using it on large areas of skin and/or for a long time (especially in children).
Due to the fact that in children the ratio of surface area to body weight is greater than in adults, children are at greater risk of suppressing the function of the hypothalamic-pituitary-adrenal axis and developing Cushing's syndrome when using any topical corticosteroids.
Long-term use of GCS in children can lead to disturbances in their growth and development.
Children should receive the minimum dose of the drug sufficient to achieve the effect.
special instructions
When used on large areas of skin for a long time, especially when using occlusive dressings, systemic action of GCS may develop. Given this, patients should be monitored for signs of suppression of the hypothalamic-pituitary-adrenal axis and the development of Cushing's syndrome.
Avoid contact of the drug with the eyes.
Elocom® ointment contains propylene glycol stearate, which may cause irritation at the site of application. In such cases, use of the drug should be discontinued and appropriate treatment should be prescribed.
It should be borne in mind that GCS can change the manifestations of some skin diseases, which can complicate the diagnosis. In addition, the use of GCS may cause a delay in wound healing.
With long-term therapy with GCS, sudden cessation of therapy can lead to the development of rebound syndrome, manifested in the form of dermatitis with intense redness of the skin and a burning sensation. Therefore, after a long course of treatment, the drug should be discontinued gradually, for example, by switching to an intermittent treatment regimen before stopping it completely.
Any of the side effects described with systemic use of GCS, including suppression of adrenal function, may also occur with topical use, especially in children.
Use in pediatrics
Due to the fact that in children the ratio of surface area to body weight is greater than in adults, children are at greater risk of suppressing the function of the hypothalamic-pituitary-adrenal axis and developing Cushing's syndrome when using any topical corticosteroids.
Long-term use of GCS in children can lead to disturbances in their growth and development.
Children should be prescribed the minimum dose of the drug sufficient to achieve an effect.
Impact on the ability to drive vehicles and operate machinery
The effect of the drug Elocom® on the ability to drive vehicles or moving machinery has not been noted.
Elokom, ointment 0.1% tube 15g (Schering-Plough Labo N.V., BELGIUM)
Composition and release form.
| mometasone furoate | 1 mg |
| excipients: hexylene glycol; phosphoric acid; propylene glycol stearate; stearyl alcohol; cetyl stearyl ether; ceteareth-20; titanium dioxide; aluminum octenyl succinate, obtained from starch; white wax; white Vaseline; purified water |
in tubes of 15 g; 1 tube in a cardboard box.
| Ointment | 1 g |
| mometasone furoate | 1 mg |
| excipients: hexylene glycol; phosphoric acid; propylene glycol stearate; white wax; white Vaseline; purified water |
in tubes of 15 g; 1 tube in a cardboard box.
| Lotion | 1 ml |
| mometasone furoate | 1 mg |
| excipients: 40% isopropyl alcohol; propylene glycol; hydroxypropylcellulose; sodium phosphate; water. May also contain phosphoric acid and sodium hydroxide to establish a pH value of approximately 4.5 |
in bottles of 20 ml;
1 bottle in a cardboard box. Pharmachologic effect. Antipruritic, anti-inflammatory, antiexudative.
The mechanism of action appears to involve inducing the release of proteins that inhibit phospholipase A2 and are collectively known as lipocortins. These proteins are thought to control the biosynthesis of potent inflammatory mediators such as PG and LT by inhibiting the release of their common precursor, arachidonic acid.
Carcinogenesis, mutagenesis.
Genetic toxicity studies of mometasone furoate, including the Ames test, the murine lymphoma test, and the micronucleus test, showed no evidence of mutagenicity.
Long-term animal experiments aimed at assessing the carcinogenic effect of the drug have not been conducted.
Pharmacokinetics.
The extent to which topical glucocorticosteroids penetrate the skin depends on many factors, including the composition of the drug and the integrity of the epidermal barrier. Inflammation and other processes occurring in the skin can lead to increased penetration of the drug through the skin. With a single local application to intact skin (without an occlusive dressing), approximately 0.7% of the ointment dose and approximately 0.4% of the cream are detected in the blood after 8 hours. There is reason to believe that the level of absorption of the glucocorticosteroid in the dosage form of the lotion is also insignificant.
Indications.
Weakening and elimination of inflammation and itching in dermatoses amenable to glucocorticosteroid therapy in adults and children over 2 years of age.
Contraindications.
Hypersensitivity.
Use during pregnancy and breastfeeding.
There have been no adequate, well-controlled studies of the teratogenic potential of mometasone furoate when used during pregnancy. The use of Elokom cream, ointment or lotion during pregnancy is possible only if the expected benefits of treatment for the mother outweigh the potential risk to the fetus.
When used systemically, glucocorticosteroids appear in breast milk, which can lead to slower growth of the child, an impact on the endogenous synthesis of glucocorticosteroids and other adverse effects. There is no evidence that systemic absorption of glucocorticosteroids when applied topically can lead to the appearance of detectable amounts in breast milk. However, due to the fact that many drugs are excreted in breast milk, nursing women should use Elokom cream, ointment, and lotion with caution.
Side effects.
Cream
. In controlled clinical studies on 319 patients, the incidence of adverse events associated with the use of Elokom cream was 1.6%. Burning, itching, skin atrophy were noted; There have been reports of rosacea. In controlled clinical studies in children aged 2 to 12 years (n=74), the incidence of adverse events (burning, itching, furunculosis) associated with the use of the cream was approximately 7%.
Ointment.
In controlled clinical studies on 812 patients, the incidence of adverse events associated with the use of Elokom ointment was 4.8%. Burning, tingling, itching, skin atrophy, furunculosis were noted; There have been reports of rosacea. In controlled clinical studies in children aged 2 to 12 years (n=74), the incidence of adverse events (burning, itching, furunculosis) associated with the use of the ointment was approximately 7%.
Lotion.
In clinical studies on 209 patients, the following adverse events were noted: burning (4 cases), acne (2 cases), itching (1 case). In a hypersensitivity study of 156 healthy volunteers, folliculitis occurred (4 cases).
With topical use of glucocorticosteroid drugs, the following adverse events may rarely occur in descending order of frequency: skin irritation and dryness, folliculitis, hypertrichosis, acne, hypopigmentation, perioral dermatitis, allergic contact dermatitis, skin maceration, secondary infection, stretch marks and miliaria. The likelihood of these adverse events occurring increases with the use of occlusive dressings.
Interaction.
No relevant data available.
Method of administration and dose.
Apply a thin layer of cream or ointment to the affected areas of the skin once a day.
Lotion - apply a few drops to the affected areas of the skin once a day; After application, the lotion is rubbed in with gentle movements until it disappears from the surface of the skin. For the most effective and economical use of the drug, it is necessary to bring the spout of the bottle closer to the affected area of the skin and lightly squeeze the bottle.
Overdose.
When applied topically in large doses, absorption of the drug is possible in quantities sufficient to cause systemic side effects.
Precautionary measures.
Elokom cream, ointment, lotion are indicated only for dermatological use and are not intended for use in ophthalmology.
As a result of systemic absorption with local use of various glucocorticosteroid drugs, reversible suppression of the function of the hypothalamic-pituitary-adrenal system, as well as symptoms of glucocorticosteroid insufficiency after discontinuation of the drug, may occur. In patients, as a result of systemic absorption of glucocorticosteroids during systemic use, Cushing's syndrome, hyperglycemia and glycosuria may also develop.
Patients receiving topical corticosteroids for the treatment of large areas of skin or under occlusive dressings should be periodically monitored for signs of suppression of the hypothalamic-pituitary-adrenal axis. This can be done by performing an ACTH stimulation test, measuring morning cortisol in plasma and in media other than urine. If suppression of the hypothalamic-pituitary-adrenal system is noted, the interval between applications should be increased, or another less potent glucocorticosteroid should be used, or the drug should be discontinued. Restoration of the function of the hypothalamic-pituitary-adrenal system usually occurs soon after discontinuation of local glucocorticosteroids. Sometimes signs and symptoms of glucocorticosteroid deficiency may appear, which requires additional use of systemic glucocorticosteroids, a description of which can be found in the annotations for such drugs.
The drug Elokom can be used in children from 2 years of age, however, it should be borne in mind that the safety and effectiveness of its use in children for a period exceeding 3 weeks have not been studied.
Due to the fact that children have a larger surface area to body weight ratio than adults, children are at greater risk of suppressing the function of the hypothalamic-pituitary-adrenal axis and the occurrence of Cushing's syndrome when using any topical glucocorticosteroid drugs. For the same reason, children have a higher risk of adrenal insufficiency when treatment with topical glucocorticosteroids is discontinued. When treated with local glucocorticosteroids in children, atrophic changes in the skin occur more easily, up to the appearance of atrophic stripes. The risk of suppression of the hypothalamic-pituitary-adrenal system in children increases when glucocorticosteroids are applied to an area of more than 20% of the body surface.
There are reports of suppression of the function of the hypothalamic-pituitary-adrenal system, the appearance of Cushing's syndrome, growth retardation, delayed weight gain and intracranial hypertension in children with topical use of various glucocorticosteroid drugs. Manifestations of adrenal insufficiency include low plasma cortisol and lack of response to ACTH stimulation. Intracranial hypertension leads to bulging fontanelles, headache, and bilateral papilledema.
Elokom ointment and cream should not be used to treat dermatitis caused by wearing diapers.
Ointment, cream and lotion should not be used under occlusive dressings unless prescribed by a doctor. Ointment and cream should not be applied to children on areas of skin that are under diapers or under waterproof underpants (occlusive dressing effect). The ointment and cream are not intended for use on the face or in the groin and armpit areas.
If irritation occurs, use of Elokom should be discontinued and appropriate treatment prescribed. Allergic contact dermatitis with corticosteroids is usually diagnosed by evidence of treatment failure, which should be confirmed by skin testing.
If a concomitant skin infection develops, an appropriate antifungal or antibacterial agent should be used. If a positive response to treatment is not achieved quickly, use of the drug should be suspended until the infection is eliminated.
As with other glucocorticosteroids, use of Elokom cream, ointment and lotion should be discontinued once cured. If there is no improvement within 2 weeks after starting therapy, the diagnosis may need to be clarified.
Manufacturer.
Elokom, ointment, cream, lotion - Schering-Plough Labo N.B., Belgium.
Elokom lotion - Schering-Plough S.p.A., Comazzo, Italy.