Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Hydrolysis of salts. Aqueous solution environment: acidic, neutral, alkaline
Hydrolysis of salts. Aqueous solution environment: acidic, neutral, alkaline
According to the theory of electrolytic dissociation, in an aqueous solution, solute particles interact with water molecules. Such an interaction can lead to a hydrolysis reaction (from the Greek hydro
- water,
lysis
- decay, decomposition).
Hydrolysis is the reaction of metabolic decomposition of a substance with water.
Various substances undergo hydrolysis: inorganic - salts, metal carbides and hydrides, non-metal halides; organic - haloalkanes, esters and fats, carbohydrates, proteins, polynucleotides.
Aqueous solutions of salts have different pH values and different types of media - acidic ($pH 7$), neutral ($pH = 7$). This is explained by the fact that salts in aqueous solutions can undergo hydrolysis.
The essence of hydrolysis comes down to the exchange chemical interaction of salt cations or anions with water molecules. As a result of this interaction, a slightly dissociating compound (weak electrolyte) is formed. And in an aqueous salt solution, an excess of free $H^{+}$ or $OH^{-}$ ions appears, and the salt solution becomes acidic or alkaline, respectively.
Classification of salts
Any salt can be thought of as the product of the reaction of a base with an acid. For example, the salt $KClO$ is formed by the strong base $KOH$ and the weak acid $HClO$.
Depending on the strength of base and acid, four types of salts can be distinguished.
Let's consider the behavior of salts of various types in solution.
1. Salts formed by a strong base and a weak acid.
For example, the salt potassium cyanide $KCN$ is formed by the strong base $KOH$ and the weak acid $HCN$:
${KOH}↙{\text"strong monoacid base"}←KCN→{HCN}↙{\text"weak monoacid"}$
In an aqueous salt solution, two processes occur:
1) slight reversible dissociation of water molecules (a very weak amphoteric electrolyte), which can be simplified by the equation
$H_2O{⇄}↖{←}H^{+}+OH^{-};$
2) complete dissociation of salt (strong electrolyte):
$KCN=K^{+}+CN^{-}$
The $Н^{+}$ and $CN^{-}$ ions formed during these processes interact with each other, binding into molecules of a weak electrolyte - hydrocyanic acid $HCN$, while the hydroxide - $ОН^{-}$ ion remains in solution, thereby determining its alkaline environment. Hydrolysis occurs at the $CN^{-}$ anion.
Let us write down the complete ionic equation of the ongoing process (hydrolysis):
$K^{+}+CN^{-}+H_2O{⇄}↖{←}HCN+K^{+}+OH^{-}.$
This process is reversible, and the chemical equilibrium is shifted to the left (towards the formation of the starting substances), because water is a much weaker electrolyte than hydrocyanic acid $HCN$.
$CN^{-}+H_2O⇄HCN+OH^{-}.$
The equation shows that:
a) there are free hydroxide ions $OH^{-}$ in the solution, and their concentration is greater than in pure water, therefore the salt solution $KCN$ has an alkaline environment
($pH > 7$);
b) $CN^{-}$ ions participate in the reaction with water, in this case they say that hydrolysis occurs at the anion
. Other examples of anions that react with water:
| $HCOO^{–}, CH_3COO^{–}, NO_2^{–}$ | from weak acids - formic $HCOOH$, acetic $CH_3COOH$, nitrogenous $HNO_2$ |
| $S^{2-}, CO_3^{2-}, SO_3^{2-}, PO_4^{3-}$ | from weak acids - hydrogen sulfide $H_2S$, carbonic acid $H_2CO_3$, sulfurous acid $H_2SO_3$, orthophosphoric acid $H_3PO_4$ |
Let's consider the hydrolysis of sodium carbonate $Na_2CO_3$.
${NaOH}↙{\text"strong monoacid base"}←Na_2CO_3→{H_2CO_3}↙{\text"weak dibasic acid"}$
Hydrolysis of the salt occurs at the $CO_3^{2-}$ anion.
The complete ionic equation for hydrolysis is:
$2Na^{+}+CO_3^{2-}+H_2O{⇄}↖{←}HCO_3^{-}+2Na^{+}+OH^{-}.$
Abbreviated ionic equation for hydrolysis:
$CO_2^{2-}+H_2O⇄HCO_3^{-}+OH^{-}.$
Hydrolysis products - acid salt
$NaHCO_3$ and sodium hydroxide $NaOH$.
The medium of an aqueous solution of sodium carbonate is alkaline ($pH > 7$), because the concentration of $OH^{-}$ ions in the solution increases. The acid salt $NaHCO_3$ can also undergo hydrolysis, which occurs to a very small extent and can be neglected.
To summarize what you have learned about anion hydrolysis:
a) according to the anion, salts, as a rule, are hydrolyzed reversibly;
b) the chemical equilibrium in such reactions is strongly shifted to the left;
c) the reaction of the medium in solutions of similar salts is alkaline ($pH > 7$);
d) the hydrolysis of salts formed by weak polybasic acids produces acidic salts.
2. Salts formed by a strong acid and a weak base.
Let's consider the hydrolysis of ammonium chloride $NH_4Cl$.
${NH_3·H_2O}↙{\text"weak monoacid base"}←NH_4Cl→{HCl}↙{\text"strong monobasic acid"}$
In an aqueous salt solution, two processes occur:
1) slight reversible dissociation of water molecules (a very weak amphoteric electrolyte), which can be simplified by the equation:
$H_2O{⇄}↖{←}H^{+}+OH^{-}$
2) complete dissociation of salt (strong electrolyte):
$NH_4Cl=NH_4^{+}+Cl^{-}$
The resulting $OH^{-}$ and $NH_4^{+}$ ions interact with each other to produce $NH_3·H_2O$ (weak electrolyte), while the $H^{+}$ ions remain in solution, causing its most acidic environment.
The complete ionic equation for hydrolysis is:
$NH_4^{+}+Cl^{-}+H_2O{⇄}↖{←}H^{+}+Cl^{-}NH_3·H_2O$
The process is reversible, the chemical equilibrium is shifted towards the formation of the starting substances, because water $Н_2О$ is a much weaker electrolyte than ammonia hydrate $NH_3·H_2O$.
Abbreviated ionic equation for hydrolysis:
$NH_4^{+}+H_2O⇄H^{+}+NH_3·H_2O.$
The equation shows that:
a) there are free hydrogen ions $H^{+}$ in the solution, and their concentration is greater than in pure water, therefore the salt solution has an acidic environment
($pH
b) ammonium cations $NH_4^{+}$ participate in the reaction with water; in this case, they say that hydrolysis occurs at the cation.
Multiply charged cations can also participate in the reaction with water: doubly charged
$M^{2+}$ (for example, $Ni^{2+}, Cu^{2+}, Zn^{2+}...$), except for alkaline earth metal cations,
triply charged
$M^{3+}$ (for example, $Fe^{3+}, Al^{3+}, Cr^{3+}…$).
Let us consider the hydrolysis of nickel nitrate $Ni(NO_3)_2$.
${Ni(OH)_2}↙{\text"weak diacid base"}←Ni(NO_3)_2→{HNO_3}↙{\text"strong monobasic acid"}$
Hydrolysis of the salt occurs at the $Ni^{2+}$ cation.
The complete ionic equation for hydrolysis is:
$Ni^{2+}+2NO_3^{-}+H_2O{⇄}↖{←}NiOH^{+}+2NO_3^{-}+H^{+}$
Abbreviated ionic equation for hydrolysis:
$Ni^{2+}+H_2O⇄NiOH^{+}+H^{+}.$
Hydrolysis products - main salt
$NiOHNO_3$ and nitric acid $HNO_3$.
The medium of an aqueous solution of nickel nitrate is acidic ($рН
Hydrolysis of the $NiOHNO_3$ salt occurs to a much lesser extent and can be neglected.
To summarize what you have learned about cationic hydrolysis:
a) according to the cation, salts, as a rule, are hydrolyzed reversibly;
b) the chemical equilibrium of reactions is strongly shifted to the left;
c) the reaction of the medium in solutions of such salts is acidic ($pH
d) the hydrolysis of salts formed by weak polyacid bases produces basic salts.
3. Salts formed by a weak base and a weak acid.
It is obviously already clear to you that such salts undergo hydrolysis of both the cation and the anion.
A weak base cation binds $OH^{-}$ ions from water molecules, forming a weak base
;
the anion of a weak acid binds $H^{+}$ ions from water molecules, forming a weak acid
. The reaction of solutions of these salts can be neutral, weakly acidic or slightly alkaline. This depends on the dissociation constants of the two weak electrolytes - acid and base, which are formed as a result of hydrolysis.
For example, consider the hydrolysis of two salts: ammonium acetate $NH_4(CH_3COO)$ and ammonium formate $NH_4(HCOO)$:
1) ${NH_3·H_2O}↙{\text"weak monoacid base"}←NH_4(CH_3COO)→{CH_3COOH}↙{\text"strong monobasic acid"};$
2) ${NH_3·H_2O}↙{\text"weak monoacid base"}←NH_4(HCOO)→{HCOOH}↙{\text"weak monobasic acid"}.$
In aqueous solutions of these salts, cations of the weak base $NH_4^{+}$ interact with hydroxy ions $OH^{-}$ (recall that water dissociates $H_2O⇄H^{+}+OH^{-}$), and the anions weak acids $CH_3COO^{-}$ and $HCOO^{-}$ interact with cations $Н^{+}$ to form molecules of weak acids—acetic acid $CH_3COOH$ and formic acid $HCOOH$.
Let us write the ionic equations of hydrolysis:
1) $CH_3COO^{-}+NH_4^{+}+H_2O⇄CH_3COOH+NH_3·H_2O;$
2) $HCOO^{-}+NH_4^{+}+H_2O⇄NH_3·H_2O+HCOOH.$
In these cases, hydrolysis is also reversible, but the equilibrium is shifted towards the formation of hydrolysis products - two weak electrolytes.
In the first case, the solution medium is neutral ($pH = 7$), because $K_D(CH_3COOH)=K+D(NH_3·H_2O)=1.8·10^{-5}$. In the second case, the solution medium is weakly acidic ($pH
As you have already noticed, the hydrolysis of most salts is a reversible process. In a state of chemical equilibrium, only part of the salt is hydrolyzed. However, some salts are completely decomposed by water, i.e. their hydrolysis is an irreversible process.
In the table “Solubility of acids, bases and salts in water” you will find a note: “they decompose in an aqueous environment” - this means that such salts undergo irreversible hydrolysis. For example, aluminum sulfide $Al_2S_3$ in water undergoes irreversible hydrolysis, since the $H^{+}$ ions that appear during hydrolysis at the cation are bound by the $OH^{-}$ ions formed during hydrolysis at the anion. This enhances hydrolysis and leads to the formation of insoluble aluminum hydroxide and hydrogen sulfide gas:
$Al_2S_3+6H_2O=2Al(OH)_3↓+3H_2S↑$
Therefore, aluminum sulfide $Al_2S_3$ cannot be obtained by an exchange reaction between aqueous solutions of two salts, for example, aluminum chloride $AlCl_3$ and sodium sulfide $Na_2S$.
Other cases of irreversible hydrolysis are also possible; they are not difficult to predict, because for the process to be irreversible, it is necessary that at least one of the hydrolysis products leaves the reaction sphere.
To summarize what you have learned about both cationic and anionic hydrolysis:
a) if salts are hydrolyzed both at the cation and at the anion reversibly, then the chemical equilibrium in the hydrolysis reactions is shifted to the right;
b) the reaction of the medium is either neutral, or weakly acidic, or weakly alkaline, which depends on the ratio of the dissociation constants of the resulting base and acid;
c) salts can hydrolyze both the cation and the anion irreversibly if at least one of the hydrolysis products leaves the reaction sphere.
4. Salts formed by a strong base and a strong acid do not undergo hydrolysis.
You obviously came to this conclusion yourself.
Let us consider the behavior of potassium chloride $KCl$ in a solution.
${KOH}↙{\text"strong monoacid base"}←KCl→{HCl}↙{\text"strong monobasic acid"}.$
Salt in an aqueous solution dissociates into ions ($KCl=K^{+}+Cl^{–}$), but when interacting with water, a weak electrolyte cannot be formed. The solution medium is neutral ($pH=7$), because the concentrations of $H^{+}$ and $OH^{-}$ ions in the solution are equal, as in pure water.
Other examples of such salts include alkali metal halides, nitrates, perchlorates, sulfates, chromates and dichromates, alkaline earth metal halides (other than fluorides), nitrates and perchlorates.
It should also be noted that the reversible hydrolysis reaction completely obeys Le Chatelier's principle. Therefore, salt hydrolysis can be enhanced
(and even make it irreversible) in the following ways:
a) add water (reduce concentration);
b) heat the solution, which increases the endothermic dissociation of water:
$H_2O⇄H^{+}+OH^{-}-57$ kJ,
which means that the amount of $H^{+}$ and $OH^{–}$, which are necessary for the hydrolysis of the salt, increases;
c) bind one of the hydrolysis products into a sparingly soluble compound or remove one of the products into the gas phase; for example, the hydrolysis of ammonium cyanide $NH_4CN$ will be significantly enhanced due to the decomposition of ammonia hydrate to form ammonia $NH_3$ and water $H_2O$:
$NH_4^{+}+CN^{-}+H_2O⇄NH_3·H_2O+HCN.$
$NH_3{↑}↖{⇄}H_2$
Hydrolysis of salts
| Salts that do not undergo hydrolysis | Salts subject to hydrolysis | ||
| reversible with equilibrium shift | irreversibly | ||
| left | right | ||
| $←$ | ${⇄}↖{←}$ | ${⇄}↖{→}$ | ${↑,↓}↖{→}$ |
| $C+C$ | $C+Cl$ | $Cl+C$ | $Cl+Cl$ |
| hydrolysis by anion - the solution is alkaline (pH > 7) | hydrolysis by cation - acidic solution environment (pH | hydrolysis by cation and anion - the solution environment depends on the dissociation constants of the base and acid formed during hydrolysis (neutral, weakly alkaline, weakly acidic) | |
Legend:
| $C$ | cation | strong | reasons | $↓$ insoluble compound |
| anion | acids | |||
| $Cl$ | cation | weak | reasons | $↑$ volatile compound |
| anion | acids |
Hydrolysis can be suppressed (significantly reducing the amount of salt being hydrolyzed) by doing the following:
a) increase the concentration of the dissolved substance;
b) cool the solution (to reduce hydrolysis, salt solutions should be stored concentrated and at low temperatures);
c) introduce one of the hydrolysis products into the solution; for example, acidify the solution if its environment as a result of hydrolysis is acidic, or alkalize if it is alkaline.
Meaning of hydrolysis
Hydrolysis of salts has both practical and biological significance. Even in ancient times, ash was used as a detergent. The ash contains potassium carbonate $K_2CO_3$, which hydrolyzes into anion in water; the aqueous solution becomes soapy due to the $OH^{-}$ ions formed during hydrolysis.
Currently, in everyday life we use soap, washing powders and other detergents. The main component of soap is sodium and potassium salts of higher fatty carboxylic acids: stearates, palmitates, which are hydrolyzed.
The hydrolysis of sodium stearate $C_{17}H_{35}COONa$ is expressed by the following ionic equation:
$C_{17}H_{35}COO^{-}+H_2O⇄C_{17}H_{35}COOH+OH^{-}$,
those. the solution has a slightly alkaline environment.
Salts of inorganic acids (phosphates, carbonates) are specially added to the composition of washing powders and other detergents, which enhance the cleaning effect by increasing the pH of the environment.
Salts that create the necessary alkaline environment of the solution are contained in the photographic developer. These are sodium carbonate $Na_2CO_3$, potassium carbonate $K_2CO_3$, borax $Na_2B_4O_7$ and other salts that hydrolyze at the anion.
If the soil acidity is insufficient, plants develop a disease called chlorosis. Its symptoms are yellowing or whitening of leaves, retarded growth and development. If $pH_{soil} > 7.5$, then ammonium sulfate fertilizer $(NH_4)_2SO_4$ is added to it, which helps to increase acidity due to hydrolysis of the cation occurring in the soil:
$NH_4^{+}+H_2O⇄NH_3·H_2O$
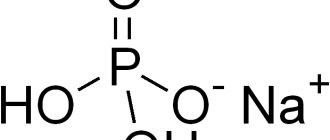
The biological role of hydrolysis of certain salts that make up our body is invaluable. For example, the blood contains sodium bicarbonate and sodium hydrogen phosphate salts. Their role is to maintain a certain reaction of the environment. This occurs due to a shift in the equilibrium of hydrolysis processes:
$HCO_3^{-}+H_2O⇄H_2CO_3+OH^{-}$
$HPO_4^{2-}+H_2O⇄H_2PO_4^{-}+OH^{-}$
If there is an excess of $H^{+}$ ions in the blood, they bind with $OH^{-}$ hydroxide ions, and the equilibrium shifts to the right. With an excess of $OH^{-}$ hydroxide ions, the equilibrium shifts to the left. Due to this, the acidity of the blood of a healthy person fluctuates slightly.
Another example: human saliva contains $HPO_4^{2-}$ ions. Thanks to them, a certain environment is maintained in the oral cavity ($pH=7-7.5$).
Content
- 1 Occurrence and production 1.1 Halite
- 1.2 Rock salt
- 1.3 Sea salt
- 1.4 Deposits
- 1.5 Production
- 2.1 In the food industry and cooking