Physical properties of FeO(II):
- black crystals;
- density 5.7 g/cm3;
- insoluble in water.
Chemical properties of FeO(II):
- it is a basic oxide;
- easily reacts with acids, forming iron salts: FeO+H2SO4 = FeSO4+H2O; FeO+2HCl = FeCl2+H2O
- easily oxidized by atmospheric oxygen: 4FeO+O2 = 2Fe2O3
- FeO(II) is obtained by reduction of FeO(III) at high temperatures: Fe2O3+H2 = 2Fe+H2O; Fe2O3+CO = 2FeO+CO2↑
Iron hydroxide Fe(OH)2(II)
Physical properties of Fe(OH)2:
- White powder;
- in air it partially oxidizes, acquiring a green tint;
- does not dissolve in water.
Chemical properties of Fe(OH)2:
- Fe(OH)2 exhibits basic properties;
- in the presence of moisture, it oxidizes, forming iron (III) hydroxide, acquiring a brown color: 4Fe(OH)2+O2+2H2O = 4Fe(OH)3
- easily reacts with acids: Fe(OH)2+2HCl = FeCl2+2H2O Fe(OH)2+H2SO4 = FeSO4+2H2O
- in concentrated alkali solutions forms ferrates (complex iron salts) when boiled: Fe(OH)2+2NaOH = Na2[Fe(OH)4]
- decomposes when heated: Fe(OH)2 = FeO+H2O
Fe(OH)2 is obtained from iron (II) salts by reacting with alkalis: FeCl2+2NaOH = Fe(OH)2+2NaCl FeSO4+2NaOH = Fe(OH)2+Na2SO4
Since Fe+2 is easily oxidized to Fe+3, all iron(II) compounds are reducing agents. Iron (II) salts also have reducing properties.
Qualitative reaction to iron (II) cation:
- to detect Fe+2, red blood salt (potassium hexacyanoferrate) is used: 3FeSO4+2K3[Fe(CN)6] = Fe3[Fe(CN)6]2↓+3K2SO4
- the presence of iron cations is judged by the resulting dark blue precipitate (Turnboole blue): 3Fe2++2[Fe(CN)6]3- = Fe3[Fe(CN)6]2↓
Literature
- Chemical Encyclopedia / Editorial Board: Knunyants I.L. and others. - M.: Soviet Encyclopedia, 1990. - T. 2. - 671 p. — ISBN 5-82270-035-5.
- Chemist's Handbook / Editorial Board: Nikolsky B.P. and others. - 2nd ed., rev. - M.-L.: Chemistry, 1966. - T. 1. - 1072 p.
- Chemist's Handbook / Editorial Board: Nikolsky B.P. and others. - 3rd ed., rev. - L.: Chemistry, 1971. - T. 2. - 1168 p.
- Ripan R., Ceteanu I.
Inorganic chemistry. Chemistry of metals. - M.: Mir, 1972. - T. 2. - 871 p.
| This is a draft article about inorganic matter. You can help the project by adding to it. |
Iron oxide Fe2O3(III)
Physical properties of Fe2O3:
- brown powder;
- can exist in three modifications: α, β, γ
- insoluble in water.
Chemical properties of Fe2O3:
- Fe2O3 exhibits amphoteric properties;
- reacts with acids: Fe2O3+6HCl = 2FeCl3+3H2O Fe2O3+3H2SO4 = Fe2(SO4)3+3H2O
- reacts with solid alkalis at high temperatures: Fe2O3+2NaOH = 2NaFeO2+H2O Fe2O3+2KOH = 2KFeO2+H2O
- reacts with sodium and potassium carbonates at high temperatures: Fe2O3+Na2CO3 = 2NaFeO2+CO2
- reacts with reducing agents: Fe2O3+2Al = 2Fe+Al2O3 3Fe2O3+CO = 2Fe3O4+CO2↑
Fe2O3 is obtained:
- pyrite firing: 4FeS2+11O2 = 2Fe2O3+8SO2↑
- decomposition of iron (III) hydroxide: 2Fe(OH)3 = Fe2O3+3H2O
Fe2O3 is contained in brown and red iron ore, which are the starting materials in the production of cast iron.
Oxides
Iron (II) oxide FeO
Iron (II) oxide is a black crystalline substance that is insoluble in water and exhibits the properties of a base. Iron (II) oxide interacts with solutions, melts and other compounds.
- With acids
FeO + 2 HCl ⟶ FeCl2 + H2O
- With air oxygen
4 FeO + O2 ⟶ 2 Fe2O3
- With hydrogen
Fe2O3 + H2 ⟶ 2 Fe + H2O
The oxide is obtained by the following reaction:
Fe2O3 + CO ⟶ 2 FeO + CO2
Iron hydroxide Fe(OH)3(III)
Physical properties of Fe(OH)3:
- substance of loose consistency of red-brown color.
Chemical properties of Fe(OH)3:
- Fe(OH)3 is a weak base;
- Fe(OH)3 exhibits amphoteric properties with a predominance of basic ones;
- reacts with dilute acids to form salts: Fe(OH)3+3HCl = FeCl3+3H2O
- reacts with concentrated solutions of alkalis upon prolonged heating to form stable hydroxo complexes: Fe(OH)3+3NaOH = Na3[Fe(OH)6]
- when heated, it decomposes to form iron (III) oxide: 2Fe(OH)3 = Fe2O3+3H2O
- Fe(OH)3 is obtained from iron (III) salts when they react with alkalis: Fe(OH)3+3NaOH = Fe(OH)3↓+3NaCl
Since, under the influence of reducing agents, Fe+3 is converted into Fe+2, all iron compounds with an oxidation state of +3 are oxidizing agents: 2Fe+3Cl3+2KI-1 = 2Fe+2Cl2+2KCl+I20
Qualitative reactions to iron (III) cation:
- Fe+3 cations are detected by the action of yellow blood salt (potassium hexacyanoferrate) - the reaction occurs with the precipitation of Prussian blue (dark blue precipitate): 4Fe+3Cl3+3K4[Fe(CN)6]-4 = Fe4[Fe(CN)6 ]3↓+12KCl
- Fe+3 cations are detected by ammonium thiocyanate (as a result of the reaction, red iron thiocyanate is formed): Fe+3Cl3+3NH4CNS- ↔ Fe(CNS)3+3NH4Cl
Iron [III] hydroxide polymaltosatum (Ferri (III) hydroxydum polymaltosatum)
Doses and timing of treatment depend on the degree of iron deficiency. The daily dose can be divided into several doses or taken once.
Inside.
Adults.
The tablets should be chewed or swallowed whole during or after meals. The daily dose can be taken in one dose. Treatment of clinically significant deficiency: 1 tablet 1-3 times a day for 3-5 months until hemoglobin concentration normalizes. Reception is continued for several months to restore iron reserves in the body (one tablet per day). pregnant women - 1 tablet 2-3 times a day (until hemoglobin content normalizes), followed by 1 tablet per day until delivery; for the treatment of latent iron deficiency and the prevention of iron deficiency - 1 tablet per day.
Drops can be mixed with fruit and vegetable juices or artificial nutritional mixtures without fear of reducing the activity of the drug. 1 ml (20 drops) contains 176.5 mg of iron hydroxide polymaltose complex (50 mg of elemental iron), 1 drop - 2.5 mg of elemental iron. To treat clinically pronounced iron deficiency, adults are prescribed 40-120 drops per day, pregnant women - 80-120 drops per day. The duration of treatment is at least 2 months. With clinically pronounced iron deficiency, normalization of hemoglobin is achieved only 2-3 months after the start of treatment. To restore its internal reserves, administration in prophylactic doses is continued for several months. To treat latent iron deficiency, adults are prescribed 20-40 drops per day, pregnant women - 40 drops per day. Prevention of iron deficiency: adults - 4-6 drops per day, pregnant women - 6 drops per day.
1 ml of syrup contains 10 mg of iron. For the treatment of clinically pronounced deficiency, adults and lactating women are prescribed 10-30 ml per day, pregnant women - 20-30 ml per day; for the treatment of latent iron deficiency - 5-10 ml per day and 10 ml per day, respectively. To prevent iron deficiency in pregnant women, the drug is prescribed in a dose of 5-10 ml per day.
Children.
Treatment of iron deficiency anemia of various origins and latent iron deficiency in infants and young children: children of the first year of life - 6-10 drops per day; at the age of 1-12 years - 10-20 drops per day.
Treatment of clinically significant iron deficiency. Inside. Premature infants - 1-2 drops per 1 kg of body weight daily for 3-5 months, up to 1 year - 10-20 drops per day; 1-12 years - 20-40 drops per day. The duration of treatment is at least 2 months. Normalization of hemoglobin is achieved only 2-3 months after the start of treatment. To restore internal iron reserves, preventive doses are continued for several months.
Prevention of iron deficiency. Inside. Up to 1 year - 2-4 drops per day; 1-12 years - 4-6 drops per day.
Treatment of clinically significant iron deficiency. Up to 1 year - 2.5-5 ml per day (25-50 mg of iron); 1-12 years - 5-10 ml per day.
Treatment of latent iron deficiency. 1-12 years - 2.5-5 ml per day.
Intramuscularly.
Adults.
The injection solution is intended for intramuscular administration only. The injection technique is of great importance. Due to incorrect administration of the drug, pain and staining of the skin at the injection site may occur. Instead of the generally accepted one, a ventrogluteal injection (into the upper outer quadrant of the gluteus maximus muscle) is recommended. Only undamaged ampoules can be used. If a precipitate forms, the solution is unsuitable for use. After opening the ampoule, it should be administered immediately.
The length of the needle should be at least 5-6 cm. The lumen of the needle should not be too wide. For children and adults with low body weight, the needles should be shorter and thinner.
According to Hochstetter
The injection site is determined as follows: point A is fixed along the line of the spinal column at a level corresponding to the lumbar-iliac joint. If the patient lies on the right side, then the middle finger of the left hand is placed at point A. The index finger is moved away from the middle finger so that it is under the line of the iliac crest at point B. The triangle located between the proximal phalanges, middle and index fingers is the injection site.
Instruments are disinfected in the usual way.
Before inserting the needle, move the skin about 2 cm in order to properly close the puncture channel after removing the needle. This prevents the injected solution from penetrating the subcutaneous tissue and staining the skin. The needle is positioned vertically relative to the skin surface, at a greater angle to the point of the iliac joint than to the point of the femoral joint. After the injection, the needle is slowly removed and the area of skin adjacent to the injection site is pressed with your finger for about 5 minutes. After administering the drug, the patient needs to move.
Children.
The effectiveness and safety of intramuscular use have not been studied.
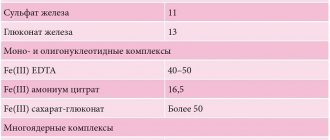
Iron salts
- Salts in which iron has an oxidation state of +2 (FeCl2, FeSO4) have reducing properties: iron sulfate FeSO4 is used as fungicides, a wood preservative, and as a component of electrolytes;
- Ferric chloride FeCl2 is used to obtain iron (III) chloride, as a catalyst in organic synthesis.
- Iron sulfate Fe2(SO4)3 is used for water purification, for the production of alum, as a component of electrolytes;
Top of page
Position in the periodic table and atomic structure
Iron is located in group 8, a secondary subgroup. This element has 26 electrons distributed over 4 levels. Electronic configuration: 1s22s22p63s23p63d64s2. Its atom is characterized by pre-filling of the s-sublevel, so in reality the formula is as follows: 1s22s22p63s23p64s23d6. Iron is a d-element. Due to this, the main oxidation states of iron are: 0, +2, +3.
Physical properties
Iron has all the properties of metals:
— plasticity (malleability);
— electrical conductivity;
— thermal conductivity;
- metallic luster;
- high melting point (15390C).
Many properties are within optimal limits, so iron is often used in the manufacture of various metal products. Alloys are made to change certain characteristics.
Basic iron alloys
Metals differ in some parameters. For example, some have a lower melting point, others have greater strength, and others are the most electrically conductive. Sometimes, to change the properties of a metal, it is alloyed with non-metals (most often carbon). The most common alloy is steel. The basis of the alloy is iron and carbon; in addition to them, various metals (alloying components) can be added that change the properties of the alloy.
Table. Iron alloys and their properties
The following can be used as alloying metals:
- Cr – chromium
- Mo – molybdenum
- Ni – nickel
- Si – silicon
- Cu – copper
- W – tungsten
- Al – aluminum
- Mn – manganese
- Ti – titanium
- Nb – niobium
- Co - cobalt
The alloying components of cast iron are non-metals: manganese, silicon, sulfur, phosphorus and some metals (aluminum, chromium).
Methods of obtaining
The main method of obtaining iron is isolation from mineral ores. The domain process is considered to be the main one. Iron is released in several stages.
Blast furnace
Table. Stages of the blast furnace process
Open hearth furnace
To reduce the content of impurities in cast iron, the resulting material is sent to an open-hearth furnace. This is a smelting plant. The process of increasing the proportion of iron occurs in three stages:
- Melting. A large amount of FeO is formed here.
- Oxidation. C+ FeO = Fe+CO. As a result of the reaction, the proportion of carbon decreases.
- Deoxidation. Oxidation of remaining FeO with aluminum, ferromanganese or ferrosilicon.
Electric oven
The installation is designed to produce alloy steel. The installation is heated to high temperatures (the figure depends on the final alloy) and an oxidizing material (nichrome, fechral, etc.) is added.
Receipt
Iron (II) hydroxide can be obtained in the form of a precipitate in exchange reactions of solutions of iron (II) salts with alkali, for example:
FeSO4 + 2KOH ⟶ Fe(OH)2↓ + K2SO4
The formation of iron (II) hydroxide is one of the stages of iron rusting:
2Fe + 2H2O + O2 ⟶ 2Fe(OH)2 Also, iron (II) hydroxide can be obtained by electrolysis of a solution of alkali metal salts (for example, sodium chloride) with stirring. First, an iron salt is formed, which, when reacted with the resulting sodium hydroxide, gives iron hydroxide. To obtain divalent hydroxide, electrolysis must be carried out at a high current density. General reaction: Fe + 2H2O ⟶ Fe(OH)2↓ + H2
Finding iron in nature
In nature, iron is found in ores. They may differ in the content of iron and other impurities. The main iron ores include: magnetite, hematite, pyrite.
Magnetite (magnetic iron ore)
Chemical formula – FeO·Fe2O3. Various metals and their oxides can be mixed with the main oxide. Zones of magnetite deposits lead to the formation of magnetic anomalies - areas of the Earth where magnetic instruments point not to the poles, but to this deposit. For this reason, it is useless to use compasses and electronic devices in these areas.
Magnetite is mined in the Chelyabinsk region, on the Kola Peninsula, the Southern Urals, and in Ukraine (Mount Krivoy Rog).
Iron ore mining crater in the Kursk Magnetic Anomaly. Source
This ore is the main one for obtaining iron, since its content in it is 72.4%. In the form of a mineral, it is used as a weighting agent for clay solutions during drilling.
Pyrite
Chemical formula - FeS2 (sulfur or iron pyrite). May contain Mn, Ni, Co impurities. This is a yellow mineral. Due to its external similarity, it was often confused with gold, which is why this mineral is often called fool's gold. Although, native gold is often contained in pyrite as impurities and is even embedded in the gold crystal lattice.
It is considered one of the most common sulfides. Pyrite deposits are located in all geothermally active zones, as well as in the bottom sediments of the Black Sea.
Upon contact with air, it oxidizes to limonite (FeOOH·(Fe2O3·nH2O).
Pyrite is used to produce sulfuric acid, hydrogen sulfide, or in the construction industry as an additive to cement.
Hematite
The chemical formula is Fe2O3. Since ancient times, the mineral has been used to make paints, ritual jewelry and medicines. Currently it is the main source of cast iron.
Main deposits:
- Ukrainian (Krivbass);
- Mikhailovskoe (KMA);
- Kolatselga adits;
- Baikal field;
- Alpine;
- Let's party.
In addition to minerals, there is also meteorite iron. This is a form of metal that came to Earth from space. During passage through the dense layers of the atmosphere, all impurities of the meteorite burn up. This iron is considered the purest. It is practically not subject to corrosion.
Mixed oxide Fe3O4
An interesting iron compound is a mixed oxide. Its peculiarity is that in one crystal lattice there are two iron ions at once - +2 and +3. Because they form a single complex, they are often written as a single oxide. In fact, it is a crystalline hydrate of two oxides: FeO⋅Fe2O3. It is characterized by the following reactions:
- Decomposition: Fe3O4 → 3Fe + 2O2
- Reaction with dilute acid: Fe3O4 + 4 H2SO4 → Fe2(SO4)3 + FeSO4 + 4 H2O
- Oxidation: 4Fe3O4 + 2O2 →6Fe2O3
- Fusion: Fe3O4 + 14NaOH → Na4FeO3 + 2Na5FeO4 + 7H2O
- Proportionation: Fe + Fe3O4 → 4FeO
- Reduction with hydrogen to iron and water.