Altevir (interferon alfa 2-b) (amp. 5 million IU/ml 1 ml No. 5)
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Interferon alpha-2b human recombinant + diphenhydramine
Compound
Injection. 5 ampoules of 1 ml. 1 ml of solution contains: Active substance: 1 million IU of human recombinant interferon alpha-2b; Excipients: sodium acetate, sodium chloride, ethylenediaminetetraacetic acid disodium salt, Tween 80, dextran 40, water for injection.
pharmachologic effect
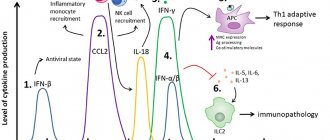
Altevir has antiviral, immunomodulatory, antiproliferative and antitumor effects. Interferon alpha-2b, interacting with specific receptors on the cell surface, initiates a complex chain of changes inside the cell, including the induction of the synthesis of a number of specific cytokines and enzymes, and disrupts the synthesis of viral RNA and viral proteins in the cell. The result of these changes is nonspecific antiviral and antiproliferative activity associated with the prevention of viral replication in the cell, inhibition of cell proliferation and the immunomodulatory effect of interferon. Interferon alpha-2b stimulates the process of antigen presentation to immunocompetent cells, has the ability to stimulate the phagocytic activity of macrophages, as well as the cytotoxic activity of T cells and “natural killer” cells involved in antiviral immunity. Prevents cell proliferation, especially tumor cells. It has an inhibitory effect on the synthesis of some oncogenes, leading to inhibition of tumor growth.
Indications for use
Altevir is used in complex therapy in adults: for chronic viral hepatitis B without signs of liver cirrhosis, for chronic viral hepatitis C in the absence of signs of liver failure (monotherapy or combination therapy with ribavirin), for laryngeal papillomatosis, genital warts, for hairy cell leukemia, chronic myeloid leukemia , non-Hodgkin's lymphoma, melanoma, multiple myeloma, Kaposi's sarcoma due to AIDS, advanced kidney cancer.
Mode of application
Subcutaneously, intramuscularly, intravenously. Treatment must be started by a doctor. Further, with the permission of the doctor, the patient can administer the maintenance dose to himself (in the case of subcutaneous or intramuscular administration). For chronic viral hepatitis B - subcutaneously or intramuscularly at a dose of 5–10 million IU 3 times a week for 16– 24 weeks Treatment is stopped after 3-4 months of use in the absence of positive dynamics (according to a DNA study of the hepatitis B virus). For chronic viral hepatitis C - subcutaneously or intramuscularly at a dose of 3 million IU 3 times a week for 6-12 months . In patients with a relapsing course of the disease and patients who have not previously received treatment with interferon alfa-2b, the effectiveness increases when using Altevir® in combination with ribavirin. The duration of combination therapy is at least 6 months. For patients with chronic hepatitis C with 1 genotype of the virus and a high viral load, in whom hepatitis C virus RNA is not detected in the blood serum by the end of the first 6 months of treatment, Altevir® therapy should be carried out for 12 months. Laryngeal papillomatosis - subcutaneously at a dose of 3 million IU /m2 3 times a week. Treatment begins after surgical (laser) removal of the tumor tissue. The dose is selected taking into account the tolerability of the drug. To achieve a therapeutic effect, therapy may be required for 6 months. Hairy cell leukemia - subcutaneously at a dose of 2 million IU/m2 3 times a week (for patients after splenectomy and without it). In most cases, normalization of one or more hematological parameters occurs after 1–2 months of treatment; it is possible to increase the treatment period to 6 months. This dosage regimen should be followed continuously, unless the disease rapidly progresses or symptoms of severe intolerance to the drug occur. Chronic myeloid leukemia - the recommended dose of Altevir® as monotherapy is 4-5 million IU/m2 per day s.c. daily. To maintain the white blood cell count, a dose of 0.5–10 million IU/m2 may be required. If treatment allows to achieve control of the number of leukocytes, then to maintain hematological remission the drug should be used at the maximum tolerated dose (4-10 million IU/m2), daily. The drug should be discontinued after 8–12 weeks of treatment if therapy has not led to partial hematological remission or a clinically significant decrease in the number of leukocytes. For non-Hodgkin's lymphoma, Altevir® is used as adjuvant therapy in combination with standard chemotherapy regimens. The drug is administered subcutaneously at a dose of 5 million IU/m2 for 2–3 months. The dose must be adjusted depending on the tolerability of the drug. For melanoma, Altevir® is used as adjuvant therapy in cases of high risk of relapse in adults after tumor removal. Altevir® is administered intravenously at a dose of 15 million IU/m2 5 times a week for 4 weeks, and then subcutaneously at a dose of 10 million IU/m2 3 times a week for 48 weeks. The dose must be adjusted depending on the tolerability of the drug. For multiple myeloma - subcutaneously at a dose of 3 million IU/m2 3 times a week. Altevir® is prescribed during the period of achieving stable remission. For Kaposi's sarcoma in the setting of AIDS, the optimal dose has not been established. The drug is used subcutaneously or intramuscularly at a dose of 10–12 million IU/m2 per day. If the disease stabilizes or responds to treatment, therapy is continued until tumor regression occurs or drug discontinuation is required. Kidney cancer - the optimal dose and regimen have not been established. It is recommended to use subcutaneous injection in doses of 3 to 10 million IU/m2 3 times a week.
Interaction
Drug interactions between Altevir® and other drugs have not been fully studied. Altevir® should be used with caution simultaneously with hypnotics and sedatives, narcotic analgesics and drugs that potentially have a myelosuppressive effect. When Altevir® and theophylline are prescribed simultaneously, it is necessary to monitor the concentration of the latter in the blood serum and, if necessary, change its dosage regimen. When using Altevir® in combinations with chemotherapeutic antitumor drugs (cytarabine, cyclophosphamide, doxorubicin, teniposide) increase the risk of toxic effects.
Side effect
Most often - fever, weakness (they are dose-dependent and reversible reactions, disappear within 72 hours after a break in treatment or its cessation), headache, myalgia, chills, loss of appetite, nausea. Less often - vomiting, diarrhea, arthralgia, asthenia, drowsiness, dizziness, dry mouth, alopecia, depression, suicidal thoughts and attempts, malaise, increased sweating, change in taste, irritability, insomnia, decreased blood pressure. Rarely - abdominal pain, skin rash, nervousness, itchy skin, anxiety, weight loss body, dyspepsia, tachycardia, autoimmune thyroiditis. Changes (reversible) in laboratory parameters: leukopenia, granulocytopenia, decreased hemoglobin levels, thrombocytopenia, increased activity of liver enzymes.
Contraindications
hypersensitivity to recombinant interferon alfa-2b or any of the components of the drug; history of severe cardiovascular disease (uncontrolled chronic heart failure, recent myocardial infarction, severe heart rhythm disturbances); severe renal and/or liver failure (including caused by the presence of metastases); epilepsy and/or other severe disorders of the central nervous system, especially manifested by depression, suicidal thoughts and attempts (including a history); chronic hepatitis with decompensated cirrhosis of the liver and in patients on or after previous therapy with immunosuppressants ( with the exception of the condition after completion of short-term therapy with GCS); autoimmune hepatitis and other autoimmune diseases, as well as taking immunosuppressive drugs after transplantation; thyroid disease that cannot be controlled by conventional therapeutic methods; decompensated lung diseases (including COPD); diabetes mellitus, prone to ketoacidosis; hypercoagulation (incl. thrombophlebitis, pulmonary embolism); severe myelosuppression; pregnancy; breastfeeding period.
special instructions
Before treatment with Altevir for chronic viral hepatitis B and C, it is recommended to perform a liver biopsy to assess the degree of liver damage (signs of active inflammatory process and/or fibrosis). The effectiveness of treatment of chronic hepatitis C increases with combination therapy with Altevir and ribavirin. The use of Altevir is not effective in the development of decompensated liver cirrhosis or hepatic coma. If side effects occur during treatment with Altevir, the dose of the drug should be reduced by 50% or the drug should be temporarily discontinued until they disappear. If side effects persist or reappear after a dose reduction, or progression of the disease is observed, treatment with Altevir should be discontinued. If the platelet level decreases below 50×109/l or the granulocyte level below 0.75×109/l, it is recommended to reduce the dose of Altevir by 2 times with blood test control after 1 week. If these changes persist, Altevir should be discontinued. If the platelet level decreases below 25×109/l or the granulocyte level below 0.5×109/l, it is recommended to discontinue the drug Altevir with blood test monitoring after 1 week. In patients receiving interferon alfa preparations 2b, antibodies that neutralize its antiviral activity can be detected in the blood serum. In almost all cases, antibody titers are low, their appearance does not lead to a decrease in the effectiveness of treatment or the occurrence of other autoimmune disorders. Preparation of a solution for intravenous administration: take the volume of Altevir solution required to prepare the required dose, add to 100 ml of sterile 0.9% NaCl solution and injected over 20 minutes.
Storage conditions
Cold +5
Dispensing conditions in pharmacies
On prescription
PITRS I line. Pegylated interferon beta-1a
Pegylated interferon beta-1a is human interferon beta-1a that is chemically linked to a polyethylene glycol derivative ( hence the abbreviated name peg interferon ). This change in the natural structure of the interferon protein molecule increases the stability of the substance, reduces the response of the immune system and allows you to prolong the effect of the drug.
Drugs from the group of interferons beta have proven effectiveness and safety of use in relapsing multiple sclerosis. Interferons beta have been successfully used to treat patients. However, such drugs have some disadvantages - the need for frequent injections and loss of therapeutic effectiveness as a result of the formation of antibodies. To improve the pharmacological properties, attempts have been made to chemically modify the natural structure of interferon molecules. One of these modifications was pegylation—the addition of a methoxypolyethylene glycol molecule to an interferon beta-1a molecule [1, 2].
Pegylated interferon beta-1a:
- modulates the immune system and suppresses the inflammatory process;
- reduces the number of exacerbations of multiple sclerosis;
- slows down the progression of disability;
- requires fewer injections due to prolonged action;
- does not cause an immune response from the body (the frequency of formation of neutralizing antibodies is less than 1%), unlike other interferon preparations.
Pegylated interferon beta-1a is indicated for the treatment of relapsing-remitting multiple sclerosis. The dosage of the drug is determined by the doctor individually.
The drug is a solution for subcutaneous administration. Injections can be administered at home independently, but supervision by a healthcare professional is recommended at the beginning of treatment [3].
Peginterferon has some contraindications for use:
- hypersensitivity to interferon beta or peginterferon;
- pregnancy;
- severe form of depression;
- age under 18 years;
- Use with caution in patients with impaired renal or liver function, epilepsy.
The most common side effects of pegylated interferon beta-1a are flu-like syndrome (headache, fever, chills) and post-injection reactions (itching, pain, skin irritation). Unpleasant symptoms are most severe during the first weeks of treatment and disappear as therapy continues. However, any changes in your health should be reported to your doctor so that additional medications can be prescribed to reduce discomfort. In some cases, if severe adverse reactions occur, the doctor may decide to change the drug [3].
The history of the use of medicines probably goes back as long as human civilization has existed. From the period of cave paintings of primitive people to the present day, the human intellect persistently demonstrates the conscious need to intervene in the disease through the use of medicines, the arsenal of which consists not only of herbs and minerals, but also antibiotics, antitumor drugs, proteins, cytokines, which require the highest intellectual and material costs for your production. And no matter what drug is used to treat a particular disease, it is logical that the doctor and the patient strive for maximum effectiveness of the drug used and minimization of its side effects. Ways to increase the clinical effectiveness of a medicinal substance can be different: this is a change in its chemical structure, a combination with other drugs that can enhance or, conversely, weaken the effect of the main drug. A special problem in this sense is posed by drugs with a protein or peptide structure (interferons, hormones, growth factors, cytokines, thrombolytics), since a fundamental change in the structure of their molecule as a chemical concept either eliminates their biological properties or entails an increase in the number of side effects associated with their use . At the same time, treatment with native drugs of protein or peptide nature has a number of significant disadvantages: they quickly hydrolyze in the digestive tract and therefore are used, as a rule, parenterally. The relatively short “natural” half-life of such drugs in the patient’s body suggests their repeated use to achieve the required therapeutic effect. Another serious negative factor limiting the use of native or recombinant peptide-protein drugs is their high immunogenicity and associated hypersensitivity reactions.
One of the ways to increase the effectiveness of drugs with a protein structure is the chemical modification of their molecule, which does not consist in actually changing its structure, but in a physicochemical transformation achieved by combining the native molecule with polyethylene glycol (PEG). The process of combining a native drug molecule with PEG is called “PEGylation.” Such chemical modification of pharmaceutical drugs with protein structure is specifically aimed at improving their tolerability, reducing immunogenicity, increasing their half-life and, ultimately, improving the patient’s quality of life during treatment.
PEG as a potential modifier of substances of protein and peptide nature attracted the attention of researchers back in the early 70s of the last century. Currently, PEG is approved by the US Food and Drug Administration (FDA) as a substance approved for use in medicine (drug production), food and cosmetology. PEG molecules are water-soluble polymers of ethylene oxide with two terminal hydroxyl groups. They can have different molecular weights and stereochemical structures. The mass of PEG molecules can vary between 300-4000 Daltons, and PEG macromolecules arranged in chains can form both branched and linear stereochemistry. It is the mass of PEG and its stereochemical structure that usually determine the fundamental properties of the future modified peptide substrate.
PEG can be bound to a protein in several positions, but some of them are premium. For example, most often a covalent connection with a protein occurs at the site of the nitrogen atom of the E-amino group of lysine (interferon-alpha 2a) or at the site of the imidazole group of histidine (interferon-alpha 2b). Such bonds make it possible to activate the hydroxyl groups of PEG and build a molecule in which the target sites have covalent bonds. In addition, direct covalent bonds of PEG at the position of lysine and arginine (carboxyl ends) directly prevent the cleavage of the modified molecules by trypsin.
Another of the most important features of modified PEG molecules is their high hydrophilicity, which forms fundamentally new physicochemical properties of the modified peptide. The high content of hydrogen atoms even in one PEG molecule allows it to bind to 2-3 water molecules. A similar effect entails the formation of a “water cloud” around the modified PEG-protein molecule, due to which its hydrodynamic radius significantly increases. This kind of “shield” of water around the modified molecule, on the one hand, significantly increases the solubility and bioavailability of the drug, and on the other, protects the molecule from other proteins (neutralizing antibodies, complement). Thus, PEG-modified proteins and peptides are much more protected from opsonization and active phago- and endocytosis of the cellular structures of the macroorganism. MonomethoxyPEG is most often used to link peptide molecules; its molecule has one hydroxyl group, with which the peptide binds; the other ends of the molecule contain non-reactogenic methyl groups. Examples of the formation of possible PEG bonds with molecules of the peptide structure are presented in Figure 1.
Changes in the pharmacokinetic and pharmacodynamic properties of PEG-modified peptides depend both on the mass of the PEG molecule and on specific binding sites. For example, a direct correlation has been demonstrated between the mass of the PEG molecule and the half-life of the peptide itself. Table 1 provides comparative data on the half-lives of some biologically active native proteins, peptides and their PEG conjugates. In general, longer PEG chains are associated with greater PEG-peptide conjugate half-life and pharmacological stability. Another important factor influencing the pharmacodynamics and pharmacokinetics of PEG-modified peptides is the structure of the PEG chains: the branched PEG molecule slows down the active metabolism of the drug, which also entails an increase in the time of its active circulation. The branched structure of PEG chains is also associated with a significantly lower immunogenicity of the modified drugs while maintaining their basic pharmacological properties. Similar effects can be achieved in another way - for example, by binding the peptide not with one, but with several PEG molecules with a linear chain structure. An example is interleukin-2 (IL-2). Because the IL-2 molecule is very small, it is freely filtered through the kidneys and has a very short half-life. The combination of IL-2 with PEG having a molecular weight of less than 20 kDa has virtually no effect on its pharmacodynamics, but increasing the molecular weight of PEG to 60-70 kDa significantly slows down the filtration of the PEG-IL-2 conjugate and increases its half-life and bioavailability. Speaking about specific sites of binding of PEG to the peptide, it should be emphasized that the strongest bonds with respect to possible proteolysis (most often by trypsin) are formed if the binding of the peptide to PEG occurs at the site of histidine.
Table 2 provides data on PEG-modified compounds of protein or peptide nature that are approved by the FDA for medical use or are at various stages of preclinical and clinical trials. From the data presented in it it follows that modern technologies for pegylation of biologically active protein and peptide molecules have led to a significant expansion of the scope of their present and future practical application. The emergence of PEG conjugates of proteins and peptides has made it possible to observe a number of fundamentally new pharmacodynamic and pharmacokinetic effects of known compounds. A striking example of this is the results of already conducted clinical trials of PEG-erythrocytes, PEG-interferon-alpha 2b, PEG-adenosine deaminase, PEG-soluble tumor necrotizing factor receptor-alpha.
This report examines the structure, pharmacodynamics, and clinical efficacy of the recently introduced pegylated interferon alpha analogues, PEG-interferon-alpha 2b (Pegintron; Shering Plough) and PEG-interferon-alpha 2a (Pegasys; Hoffmann La Roche). It should be emphasized that the safety profile, pharmacodynamics, pharmacokinetics and clinical effectiveness of the drugs mentioned were studied in most detail using the example of patients with chronic hepatitis C. This circumstance is extremely important, since with chronic hepatitis C, unlike, for example, chronic hepatitis B, In the near future there will be no serious alternative to interferon-alpha as a means of basic treatment. Unfortunately, the results of therapy for chronic hepatitis C with native (“short”) interferon-alpha cannot be considered satisfactory: the rate of proven response to treatment in the general population of patients with this disease did not exceed 20-22%, and in patients with HCV genotype 1b and/or liver cirrhosis – 8-10%. One of the main reasons for such disappointing results is the short half-life of the native alpha-interferon molecule, which does not provide reliable control of the kinetics of the hepatitis C virus. However, the short half-life of native alpha-interferon is not the only reason for its low effectiveness in the treatment of chronic hepatitis C. This is confirmed by the lack of significant improvement in treatment results when using daily or high-dose monotherapy regimens with native alpha interferons. Apparently, factors such as the induction of antibody formation, the immunogenic properties of the alpha-interferon molecule, the characteristics of its metabolism and some other points play no less a role than the short half-life in the ineffectiveness of these drugs.
PEG-interferon-alpha 2b (PEGINTRON)
Antiviral therapy is one of the main areas of promising use of PEGylated drugs with a peptide structure. Currently, non-pegylated interferon-alpha 2b (Intron-A), which can suppress the replication of viral DNA or RNA, is widely used for the treatment of chronic viral hepatitis B and C. With the traditional regimen of its use (3 million IU 3 times a week), the maximum concentration of the drug after subcutaneous administration is determined within 8-12 hours, and its half-life averages 6 hours. From this it clearly follows that, along with periods of stable concentration of the drug, there are also periods when its level in the blood serum and body tissues can decrease to almost undetectable values. It is logical to assume that in order to achieve an acceptable therapeutic level of interferon-alpha administered externally, a significant change in the parameters of its pharmacokinetics and pharmacodynamics is necessary. This formulation of the question predetermined the creation of a fundamentally new dosage form of interferon-alpha 2b conjugated with PEG. This drug has already passed all the necessary clinical trials and is registered for use in all leading European countries and the USA under the trade name Pegintron. Figure 2 shows the main parameters of the pharmacokinetics of native interferon-alpha 2b and Pegintron. It is obvious that Pegintron has a significantly better pharmacological profile than native interferon-alpha 2b, which is reflected in a significantly increased half-life and weakened immunogenic properties. During the development of Pegintron, it was shown that its antiviral activity in vitro is approximately 35-40% of that of native interferon-alpha 2b. At the same time, the addition of a relatively small PEG molecule with a molecular weight of 12 kDa to native interferon leads to the fact that in vivo the maximum concentration of the latter is achieved, as with the use of a non-PEGylated drug, after 8 - 12 hours, but a stable concentration is maintained for 48- 72 hours. Thus, the “effective” half-life of the drug averages about 40 hours. This pharmacological profile is ensured by administering the drug once a week, which has a beneficial effect on the quality of life of the patient receiving antiviral treatment. Another fundamentally important advantage of Pegintron over native interferon-alpha 2b is that it can be used for liver cirrhosis. Previously, patients with chronic hepatitis C at the stage of morphological cirrhosis were, as a rule, deprived of the opportunity to receive full antiviral therapy, since the administration of non-pegylated interferon-alpha could significantly worsen their condition. The structural features of the Pegintron molecule (relatively small size and linearity) make it possible to use it in this category of patients, since the drug does not require highly preserved renal hemoperfusion for complete elimination. Clinical trials of Pegintron have demonstrated its obvious advantages over native interferon-alpha in the treatment of chronic hepatitis C, both when used as monotherapy and in combination with ribavirin.
PEG-interferon-alpha 2a (PEGASIS)
Another representative of the group of pegelated interferon-alpha is the interferon-alpha analogue 2a, marketed under the trade name Pegasys. It differs from Pegintron in a slightly more complex chemical structure - interferon is connected to a branched chain of PEG, which has a higher molecular weight - 40 kDa (the total molecular weight of Pegasys is about 60 kDa), during conjugation an amide chemical bond is used at the position of the amino group of lysine, which is the strongest. This predetermined the characteristics of the pharmacodynamics and clinical effectiveness of Pegasis in patients with chronic hepatitis C. Research results allow us to consider this drug as the most effective among alpha interferons, especially in the so-called complex category of patients with HCV genotype 1b and/or high viral load, with cirrhosis liver.
Although special studies have not been conducted to compare the effectiveness of Pegintron and Pegasys in patients with chronic hepatitis C, the data available today from clinical trials of these drugs suggest a certain advantage of Pegasys both in terms of the antiviral effect and the impact on the quality of life of patients.
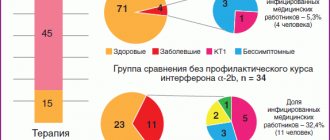
The use of Pegasys as monotherapy in patients with chronic hepatitis C on average allows achieving a stable therapeutic response in 39% of cases, while with the use of Pegintron - only in 25% (Fig. 3). Pegasys monotherapy was even more successful in the “difficult” category of patients: treatment was effective in almost a third of patients. Pegintron, when prescribed in a similar regimen, was effective only in 14% of cases (Fig. 4).
Of interest are the results of studies on the effectiveness of pegylated interferon-alpha used in combination with ribavirin, presented by Di Bisciegli (2000). As it turned out, in patients with an HCV genotype other than 1b, the treatment effectiveness when using Pegintron and Pegasys was the same (sustained response in 75% and 76% of cases, respectively), but in the group of patients with HCV genotype 1b, Pegasys was significantly superior to Pegintron - a stable response was obtained in 41% and 30% of cases, respectively (Fig. 5). Thus, in this category of patients, when conducting combination therapy for chronic hepatitis C, preference should be given to Pegasys. In patients with liver cirrhosis, the use of Pegasys in a combination treatment regimen also made it possible to achieve a significant increase in the rate of sustained therapeutic response (up to 43%; Fig. 6).
Analyzing the frequency of side effects (Fig. 7) of pegylated interferon-alpha according to the literature, it can be stated that they were observed less frequently when using Pegasys.
Noteworthy is the significant improvement in the quality of life of patients who were prescribed Pegasys compared to those who were administered native interferon-alpha 2a (Fig. 8). This is due to the good tolerability and ease of use of Pegasys. When using it, there is no need to select a dose depending on the patient’s body weight (unlike Pegintron), the drug is administered once a week and is available in the form of a finished liquid dosage form that does not require preliminary manipulations during use (unlike Pegintron).
Thus, the development and introduction of pegylated interferon-alpha drugs into the practice of combination therapy for chronic hepatitis C allows us to talk about a new “gold standard” for the treatment of this disease, and the results of their use inspire some optimism. The issue of correct selection of patients for such therapy, on which the results of treatment and the patient’s quality of life largely depend, is becoming extremely important.