pharmachologic effect
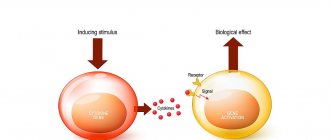
Human leukocyte interferon is a mixture of various subtypes of natural interferon alpha from human leukocytes. It has antiviral, antiproliferative, immunomodulatory and antitumor effects. The mechanism of antiviral action is to create protective mechanisms in cells not infected with the virus: changing the properties of cell membranes that prevent the virus from penetrating into the cell; initiation of the synthesis of a number of specific enzymes that prevent the replication of viral RNA and the synthesis of viral proteins. The antiproliferative effect is due to direct mechanisms that cause changes in the cytoskeleton and cell membrane, regulating the processes of differentiation and cellular metabolism, which in turn prevent the proliferation of cells, especially tumor cells. Helps modulate the expression of some oncogenes (myc, sys, ras), which allows to “normalize” neoplastic transformation of cells and thereby inhibit tumor growth. The immunomodulatory effect is due to stimulation of the activity of macrophages and natural killer cells (macrophages are involved in the process of presenting antigen to immunocompetent cells, and natural killer cells are involved in the body’s immune response to tumor cells).
What is interferon?
Interferons (IFNs) are a group of proteins that are released by cells in response to viral infection. In some cases, interferons begin to be released in response to infection by certain types of bacteria. Interferons were first discovered in 1957, when scientists Alik Isaacs and Jean Lindeman discovered that mice infected with one virus were not infected with other viruses. It was easy for scientists to conclude that in response to a viral infection, cells produce certain universal substances with antiviral activity2.
Currently, more than 20 interferons are known, which are divided into three types3,4:
- Type I – viral interferons. These include interferon alpha (IFN-α), interferon beta (IFN-β), and several other interferons. Interferons alpha and beta are produced in response to viruses, as well as bacterial components that stimulate not only the production of interferon, but also other immune mechanisms.
- Type II – immune interferon, including gamma interferon (IFN-γ). Unlike type 1 interferons, which are the first to respond to a viral infection, gamma interferon is produced at subsequent stages of infection.
- Type III – interferon lambda (IFN-λ). This type of interferon was recently discovered, and its mechanism of action resembles the first type of interferon.
Indications for use
- Hairy cell leukemia
- Chronic myeloid leukemia
- Viral hepatitis B
- Viral active hepatitis C
- Primary (essential) and secondary thrombocytosis
- Transitional form of chronic granulocytic leukemia and myelofibrosis
- Multiple myeloma
- Kidney cancer
- AIDS-related Kaposi's sarcoma
- Mycosis fungoides
- Reticulosarcoma
- Multiple sclerosis
- Prevention and treatment of influenza and acute respiratory viral infection
Advantages and disadvantages of introduced interferons
The advantages of drugs containing interferons include their availability. An important advantage of interferons is their possible use against a wide range of viruses.
The relatively high level of safety of interferon drugs allows them to be used by children, who, as is known, often suffer from influenza and other acute respiratory viral infections.
However, along with the advantages, such drugs also have their disadvantages. Often, excessive amounts of type 1 interferon are associated with suppression of innate antibacterial immunity mechanisms8 and can also provoke a decrease in the production of intrinsic interferon9.
Directions for use and doses
To prevent influenza and other acute respiratory viral infections, use the drug by spraying or instilling an aqueous solution. Administration by injection is strictly prohibited.
The bottle with the drug should be opened immediately before use. An opened bottle, carefully closed with a dropper made of polymer material, can be stored at a temperature of 2 to 8 ° C for up to three days.
Spraying of the drug can be done using sprayers of any system. The same dosage regimen is used for children and adults. When instilling the drug, administer 5 drops into each nasal passage 2 times a day with an interval of at least 6 hours. When treating the drug, use it by inhalation, spraying or instillation.
The same dosage regimen is used for children and adults. The most effective way is inhalation. For this purpose, inhalers equipped with electrical heating or another system are recommended. For one administration, use the contents of 3 ampoules of the drug, to which add 4 ml of water. Heat the solution to a temperature not higher than 37 °C. The drug is administered by inhalation 2 times a day with an interval of at least 6 hours. When instilled, the drug is administered 0.25 ml (5 drops) into each nasal passage after 1-2 hours, at least 5 times a day, for 2-3 days.
Human leukocyte interferon
S/c, i/m; maximum doses - intravenous drip, slowly (over 30-60 minutes). The required dose is pre-diluted with 50 ml of 0.9% NaCl solution (12 million IU).
For hairy cell leukemia, the recommended initial dose is 3 million IU per day for 6 months. If therapy is ineffective, the drug is discontinued; if positive dynamics are observed, then treatment should be continued until hematological parameters improve, and after achieving stability, therapy is carried out for another 3 months at 3 million IU 3 times a week.
For multiple myeloma, the initial dose is 3 million IU 3 times a week with a weekly increase to the maximum tolerated dose of 9-18 million IU 3 times a week. This regimen should be maintained indefinitely, except in cases where the disease develops too quickly or the patient becomes intolerant to the drug.
For non-Hodgkin's lymphoma, the initial dose is 3 million IU 3 times a week for 2 weeks, then the recommended dose is 6 million IU 3 times a week; from 14 weeks - 3 million IU 3 times a week.
Maintenance therapy after standard chemotherapy (with or without radiation therapy) - 3 million IU 3 times a week for at least 12 months.
For cutaneous T-cell lymphoma: - days 1-3 - 3 million IU/day, days 4-6 - 9 million IU/day, days 7-84 - 18 million IU/day; maintenance dose - maximum tolerated (no more than 18 million IU) 3 times a week.
For chronic myeloid leukemia and thrombocytosis in chronic myeloid leukemia: days 1-3 - 3 million IU/day, days 4-6 - 6 million IU/day, days 7-84 - 9 million IU/day, course - 8-12 weeks. After stabilizing the number of leukocytes, the frequency of administration is 3 times a week. This regimen should be maintained indefinitely, except in cases where the disease develops too quickly or the patient becomes intolerant to the drug.
For thrombocytosis in myeloproliferative diseases (except chronic myeloid leukemia): days 1-3 - 3 million IU/day, days 4-30 - 6 million IU/day.
For Kaposi's sarcoma: days 1-3 - 3 million IU/day, days 4-6 - 9 million IU/day, days 7-9 - 18 million IU/day, if tolerated, days 10-84 - up to 36 million IU/day ; maintenance dose - maximum tolerated (no more than 36 million IU) 3 times a week.
For renal carcinoma: days 1-3 - 3 million IU/day, days 4-6 - 9 million IU/day, days 7-9 - 18 million IU/day, if tolerated, days 10-84 - up to 36 million IU/day ; maintenance dose - maximum tolerated (no more than 36 million IU) 3 times a week; The duration of treatment ranges from 8-12 weeks to 16 months. In combination therapy with vinblastine - 3 million IU 3 times a week, in the second week - 9 million IU 3 times a week, then 18 million IU 3 times a week. During this period, vinblastine is administered at a dose of 0.1 mg/kg once a week. Duration of therapy is 3-12 months. In case of complete remission, treatment can be stopped 3 months after its onset.
Melanoma - 18 million IU 3 times a week for 8-12 weeks; maintenance dose - 18 million IU 3 times a week for 8-12 weeks; If there is a positive effect, therapy is continued for up to 17 months; if there is no positive effect, it is stopped.
For acute hepatitis B (mild, moderate and severe forms), 1 million IU is prescribed 2 times a day for 5-6 days, then 1 million IU/day is administered for another 5 days. If necessary, the course of treatment can be continued - 1 million IU 2 times a week for 2 weeks. The course dose is 15-21 million IU.
For chronic active hepatitis B (including delta-positive chronic hepatitis B), the recommended dose is 4.5 million IU 3 times a week for 4-6 months. If the number of markers of viral replication or hepatitis B virus surface antigen does not decrease after 1 month of treatment, then the dose should be increased to 6 million IU 3 times a week. If there is no improvement after 3-4 months, the course of treatment should be stopped.
For chronic hepatitis C, 3-6 million IU is prescribed 3 times a week, the duration of treatment is up to 3 months; maintenance dose - 3 million IU 3 times a week for another 3-9 months (to consolidate remission in patients with normalized ALT activity in plasma). If within 16 weeks from the start of therapy there is no decrease in the activity of “liver” transaminases, then treatment should be discontinued.
For genital warts, the dose is 0.1-1 million IU (depending on the area of the lesion) 3 times a week. The drug is injected with a thin needle into the base of the damaged area; the number of lesions should be counted to calculate the total simultaneously administered dose, which should not exceed 3 million IU. Each cycle of therapy includes 3 doses per week for 3 weeks. Improvement is usually observed within 4-6 weeks from the start of the first cycle of therapy. In some cases, the treatment cycle should be repeated using similar doses.
For tick-borne encephalitis (including the meningeal form), 1-3 million IU is administered 2 times a day for 10 days. Then they switch to maintenance therapy of 1-3 million IU every 2 days 5 times.
Primary and secondary thrombocytosis - 2 million IU/day 5 days a week for 4-5 weeks. If the platelet count does not decrease after 2 weeks, the dose is increased to 3 million IU/day; if there is no effect by the end of 3 weeks, the dose is increased to 6 million IU/day. For initial thrombocytopenia (less than 15 G/l), the initial dose is 0.5 million IU/day.
During the transition phase of chronic granulocytic leukemia and myelofibrosis - 1-3 million IU per day according to the regimen.
Locally. Intranasally (the contents of the ampoule are dissolved in 2 ml of distilled water - 40 drops), for the treatment of influenza and ARVI in the first hours of the disease, 3-4 drops (about 0.2 ml) are instilled into each nasal passage every 15-20 minutes for 3-4 hours , then 4-5 times a day for 3-4 days. For the prevention of influenza and ARVI - 5 drops 2 times a day (while the risk of infection remains).
In the conjunctival sac: the contents of the ampoule are dissolved in 1 ml of sterile distilled water. In the acute stage of the disease, 2-3 drops (about 0.1 ml) are instilled into each eye 3-10 times a day in combination with symptomatic treatment. As the inflammatory process subsides, the number of instillations is reduced to 5-6 times a day. The course of treatment is 12-14 days, if necessary - up to 30 days.
Rectally. For hemorrhagic fever with renal syndrome and secondary cellular immunodeficiency states, a daily dose of 120 thousand IU is prescribed (1 suppository 4 times a day with a break of 6 hours) or 60 thousand IU 2 times a day with a break of 8 hours). The course of treatment is 7-14 days. For acute hepatitis B in children, it is prescribed according to the following scheme: from days 1 to 3 - 40 thousand IU 2 times a day, from days 4 to 7 - 40 thousand IU once a day, from days 8 to 14 - 40 thousand. IU every other day. The course of treatment is no more than 14 days. Course dose - 560 thousand IU
Contraindications
- Hypersensitivity, severe heart disease (including a history), acute myocardial infarction, severe dysfunction of the liver, kidneys or hematopoietic system, epilepsy and/or other disorders of the central nervous system.
- Chronic hepatitis against the background of decompensated cirrhosis of the liver
- Chronic hepatitis in patients receiving or recently receiving immunosuppressant therapy (except for short-term pre-treatment with steroids)
- Pregnancy
- Breastfeeding (breastfeeding should be stopped)
- Childhood