Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Experience in the treatment of chronic adenoiditis in preschool children
Introduction
Non-communicable diseases are on the rise worldwide [1]. Diseases of the organs of the lymphopharyngeal ring occupy 1st place in prevalence among all ENT diseases in pediatric otorhinolaryngology. Chronic adenoiditis is characterized by difficult passage of air through the nose due to the proliferation of hypertrophied tonsil tissue. In this case, nasal breathing is blocked, ventilation and normal function of the pharynx are disrupted, and congestion occurs in the nose and paranasal sinuses. Chronic inflammation of the nasal mucosa develops, and viscous mucus accumulates in the nasal lumen. These changes lead to brain hypoxia and a deterioration in the child’s general condition (persistent reflex cough at night, difficulty waking up in the morning, unrelieved sleep, headaches). As the disease progresses, the lymphoid tonsils gradually completely close the openings of the auditory tubes, resulting in decreased hearing, hearing loss, and worsening chronic otitis media and sinusitis. The child begins to lag behind in development, does poorly at school, and speech development is delayed [2–5]. Currently, there is no doubt that toxins, both endogenous and exogenous, make a significant contribution to the occurrence of chronic inflammation, and, therefore, detoxification of organs and elimination of toxins is necessary, which will ensure autoregulation [6–9]. Activation of the organs responsible for drainage is also necessary, especially if they are involved in the inflammatory process, as happens with the mucous membranes with adenoiditis [6, 10, 11]. Children with chronic adenoiditis, as a rule, have a history of frequent episodes of acute respiratory infection (ARI) [12, 13]. When selecting pathogenetic therapy for chronic inflammation, it is advisable to take into account the leading changes in the child’s body. Thus, for the regeneration of mucous membranes, the drug Mucosa compositum is recommended, which is capable of correcting functional, organic and dysbiotic disorders in all loci of the mucous membrane, regardless of the lesion [14, 15]. For the treatment of children with hyperplasia of lymphoid tissue, the universal drainage drug Lymphomyosot is indicated [14, 15], in order to restore the mucous membranes of the nasal cavity and relieve symptoms of acute inflammation, the drug Euphorbium compositum can be used [14, 15]. Antihomotoxic therapy is characterized by high clinical effectiveness, the possibility of an individual approach, the almost complete absence of contraindications and side effects, the stability and duration of the effect obtained, environmental feasibility, the possibility of reducing the dose of allopathic drugs, and economic feasibility [16]. Most of these aspects of antihomotoxic treatment completely coincide with the requirements for pharmacological drugs and methods of therapy used in pediatrics. Considering the recurrent nature of the course of chronic adenoiditis, a decrease in the body’s ability to autoregulate cannot be ruled out. It is in such situations that therapy is necessary, based on the three basic principles of homotoxicology: detoxification, drainage, immunomodulation [14–17]. Purpose of the study:
to evaluate the effectiveness of treatment of chronic adenoiditis with antihomotoxic drugs in preschool children.
Material and research methods
A retro- and prospective study was carried out in the conditions of children's clinic No. 1 in Samara. We observed 80 children aged 4 to 6 years with a diagnosis of moderate chronic adenoiditis. The duration of the disease was 2.0±0.6 years. The criteria for inclusion of patients in the study were: • diagnosis of “chronic adenoiditis”; • average severity of the disease; • absence of signs of other infectious diseases; • completeness of the case (improvement as a result of treatment). Patients were observed in 2 groups. Group I – children who received antihomotoxic drugs (AGDs) as part of complex therapy; Group II – children who received standard treatment. In the life history of children of both groups, aggravating factors such as artificial feeding, monotonous (mainly carbohydrate) nutrition, diathesis (mainly of the allergic type), hypothermia, and allergies were noted. The effectiveness of antihomotoxic drugs (AGLS) was studied: Mucosa compositum, Lymphomyosot, Euphorbium compositum in the complex therapy of chronic adenoiditis in children in comparison with the effectiveness of generally accepted methods of therapy (local treatment with antimicrobial and anti-inflammatory drugs in the form of drops and sprays locally, antihistamines, physiotherapeutic procedures) taking into account the possibility of complications. The drug Mucosa compositum was prescribed 0.5 ampoules intramuscularly 2 times a week for 5 weeks. The drug Euphorbium compositum C was prescribed in the form of a spray, 1-2 injections 3-5 times a day during periods of exacerbations and for acute respiratory viral infections. The drug Lymphomyosot was prescribed orally, 6-8 drops, in 100 ml of water, 3 times a day for 30 days. The main criterion for assessing the effectiveness of therapy was clinically significant treatment results (clinical outcomes - CI). The choice of CT was based on an assessment of the clinical picture of the disease.
Clinical outcomes studied
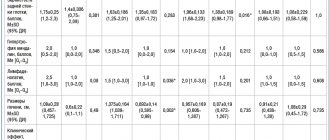
“The presence of difficulty in nasal breathing due to congestion and nasal discharge” (1) “The presence of a night cough” (2) “The presence of snoring during night sleep” (3) “The presence of congestion in both ears without hearing loss” (4) “The presence long-term (up to 2 weeks) low-grade fever - 37.7 °C" (5) "The presence of frequent acute respiratory viral infections (every 2 months) with complications in the form of tubotitis" (6) "The presence of intoxication symptoms (decreased appetite, fatigue and general weakness, restless sleep)" (7) "The presence of purulent discharge from the nasal passages during ARVI" (8). The CTs are presented in more detail in Table 1.
Key intervention indicators were calculated: NIL
– frequency of outcomes in the treatment group.
Calculated using the formula A/(A+B)
, where A is the number of patients with the presence of the studied outcome, B is the number of patients with the absence of the studied outcome;
FIC
– frequency of outcomes in the comparison group.
Calculated using the formula C/(C+D), where C is the number of patients with the presence of the studied outcome, D is the number of patients with the absence of the studied outcome; RR
– relative risk (risk ratio) – the ratio of the frequency of the studied outcomes among patients exposed to the drug.
Calculated using the formula CHIL/CHIK.
Allows you to determine the strength of the relationship between drug exposure and the outcome being studied.
In POP (increase in relative benefit - relative increase in the frequency of favorable outcomes in the treatment group compared to the control group; calculated as (CHIL - CHIC)/CHIC, given with 95% CI) ≥1.0 - high probability of outcome as a result of treatment. When LOP<1.0 the probability of outcome decreases; NNT
is the number of patients who need to be treated with a particular drug to obtain (or prevent) the outcome being studied in one patient.
Calculated using the formula 1/CHIL-CHIK; CI
– confidence interval.
Reflects sampling error. A 95% confidence interval shows that there is a 95% chance that if the experiment is repeated, the original value will be obtained; OR
– odds ratio.
Shows how many times the probability of the studied outcome in the main group is higher (or lower) than in the comparison group. Calculated using the formula (A/B)/(C/D).
OR<1 corresponds to low probability. OR>1 corresponds to high probability. OR=1 means the same result as in the comparison group. The effectiveness of the intervention was assessed using contingency tables [18–20] (see Table 1). To objectify the assessment of treatment effectiveness, methodological standards of evidence-based medicine were used. The significance of differences in the relationship between two characteristics in groups of subjects was calculated using nonparametric methods with the χ2 criterion [18, 19, 20].
Research results and discussion
Key intervention indicators are presented in Table 2 and Figure 1.
Analysis of outcomes 1–5
For outcomes 1–5, results of similar significance were obtained.
Therefore, we present a group analysis of the effectiveness of AGSTL based on these outcomes. As can be seen from the table and figure, in children receiving AGLS, adverse outcomes were observed significantly less frequently than in the group of patients receiving standard treatment. NCI values for all CIs ranged from 8.5% to 17.5%, while NIC values ranged from 52.5% to 77.5%. The reliability of statistical differences is confirmed by the values of the χ2 criterion and the p value. The absolute risk reduction and the corresponding NNT value in outcomes 1–5 is equal to 2 with a CI of 0–9, which means that every 3rd patient treated with AHL can expect a positive treatment outcome. The RR indicator (0.10–0.23 with CI 0.03–0.44) shows a lower probability of adverse outcomes in the main group of patients, since it is significantly lower than one. OR 0.06–0.19 (CI 0.02–0.21; 0.06–0.51) significantly (p≤0.005) shows that the risk of an unfavorable outcome in the treatment of AHF decreases in different CTs in 5–12 once. Thus, the significant effectiveness of AGLS for ARVI in children has been shown according to the CI: “The presence of difficulty in nasal breathing due to congestion and nasal discharge” (1); “Presence of night cough” (2); “Presence of snoring during night sleep” (3); “Presence of congestion in both ears without hearing loss” (4); “Presence of long-term (up to 2 weeks) low-grade fever – 37.7 °C” (5). Analysis of outcome 7
Based on the outcome “Presence of intoxication symptoms” (7), we can note the highest effectiveness of the use of AGLS.
A positive result was obtained in 90% of cases (10% negative). In the comparison group, only 17.5% of children achieved a positive treatment result (χ2–39.4; p=0.0005 - the difference is statistically significant). The RR was significantly less than one (0.12, CI 0.04–0.29), indicating the lowest likelihood of an adverse outcome when using these drugs. The values of the OR indicator are much lower than one (OR – 0.02 with CI 0.01–0.10) indicate that the risk of intoxication syndrome when using AHFLS is reduced by 50 times. The number of patients who need to be treated in order to prevent the development of one unfavorable outcome was equal to 1 (CI 0–5), which means that a favorable outcome is observed in every second patient, and indicates the high effectiveness of AHF for this disease. Noteworthy are the narrow limits of the CI of the NNT indicator, which confirms its high clinical and statistical significance. The high effectiveness of AGLS in relation to intoxication syndrome is apparently due to the presence in the regimen of the drug Lymphomyosot with a pronounced drainage mechanism. Analysis of outcomes 6 and 8
Comparison of the effectiveness of AGLS therapy and standard treatment according to the CT “Presence of frequent acute respiratory viral infections with complications in the form of tubotitis” (6) and “Presence of purulent discharge from the nasal passages during acute respiratory viral infections” (8) showed that, in general, the effectiveness of the use of AGLS for CI6 and CI8 is lower than for other CIs. RR (0.41 with CI 0.17–0.92 for CI6; 0.53 with CI 0.23–1.18 for CI8) and OR (0.29 with CI 0.09–0.89 for CI6 ; 0.42 with CI 0.14–1.26 for CI8) is higher than for other outcomes. That is, the likelihood of purulent complications when using AGLS is reduced only by 2.5–3.0 times. To prevent the development of one negative outcome, it is necessary to treat 4 patients for CI6 and 6 patients for CI8, which is significantly more than for other outcomes. The absence of significant differences in key indicators of the intervention was shown (χ² = 4.82 and 2.2; p = 0.03 and 0.14 - the difference is not statistically significant). The effectiveness of the treatment regimens under consideration in relation to the syndromes “presence of frequent acute respiratory viral infections with complications in the form of tubotitis” and “presence of purulent discharge from the nasal passages during acute respiratory viral infections” can be assessed as equal, which may be due to the lack of direct antibacterial action in the considered AGLS.
conclusions
This study showed that the use of AGLS in conjunction with conventional therapy for chronic adenoiditis can be considered effective. The used AGLS (Mucosa compositum, Lymphomyosot, Euphorbium compositum) were well tolerated and had no adverse reactions. AGLS in this study contributed to detoxification, which makes it possible not to use additional treatment for this purpose. Complex homeopathic preparations Mucosa compositum, Lymphomyosot, Euphorbium compositum can be considered as an alternative to standard therapy for moderate chronic adenoiditis in children and are recommended for the treatment of this pathology.