Pharmacology
Pharmacological action – inhibiting bone resorption.
The effect of ibandronate on bone tissue is due to its affinity for hydroxyapatite, a component of the mineral matrix of bone. Suppresses the activity of osteoclasts, reduces bone resorption. Has an inhibitory effect on tumor osteolysis. The inhibitory effect on tumor osteolysis and especially on hypercalcemia of tumor genesis is manifested in a decrease in the concentration of calcium in the serum and a decrease in the excretion of calcium in the urine. Regulates calcium levels in blood and bone tissue. Increases bone mass, especially in the spine.
A single infusion of 2 mg of the drug in patients with osteolytic hypercalcemia caused by malignant tumor growth, or with humoral tumor hypercalcemia for 7 days leads to a stable (for several weeks) normalization of calcium levels in the blood serum. When the dose is increased to a maximum of 6 mg, the clinical effectiveness increases slightly. In doses significantly higher than pharmacologically effective ones, it does not affect the mineralization of bone tissue. In women with postmenopausal osteoporosis, four times intravenous administration every 3 months was accompanied by an increase in bone mass in the spine by 6.25%, and in the proximal femur by 2.96%.
After a single two-hour infusion of 6 mg, Cmax is 328 ng/ml, after a single intravenous bolus injection of 2 mg - 246 ng/ml. After oral administration, it is rapidly absorbed in the upper gastrointestinal tract. Tmax - 0.5–2 hours (after administration on an empty stomach), absolute bioavailability - 0.6%. Simultaneous consumption of food or drinks (except pure water) reduces bioavailability by 90%. Eating or drinking 30 minutes after taking ibandronic acid reduces its bioavailability by 30%. When taking ibandronic acid 60 minutes before meals, no significant decrease in bioavailability is observed. Bioavailability is reduced to 75% when ibandronic acid is taken 2 hours after a meal. The concentration of ibandronic acid in plasma increases proportionally to the dose when administered intravenously (at a dose of up to 6 mg) or when administered orally (at a dose of 100 mg). The concentration in the blood decreases quickly and reaches 10% of Cmax 3 hours after intravenous administration and 8 hours after oral administration. Plasma protein binding is about 90%. After entering the systemic circulation, 40–50% of ibandronic acid quickly penetrates bone tissue and accumulates in it or is excreted unchanged in the urine. There is no evidence that ibandronic acid is metabolized. The apparent final volume of distribution is 90 l. The drug that is not absorbed after oral administration is excreted unchanged in the feces. Removal is biphasic. Terminal T1/2 - 10-60 hours. Total clearance - 130 ml/min, renal clearance - 88 ml/min, volume of distribution - 150 l.
Ibandronic acid-Vista tablets 150 mg, 3 pcs.
Patients with disorders of bone and mineral metabolism
Before starting treatment with Ibandronic acid-Vista, hypocalcemia and other disorders of bone tissue and mineral metabolism must be corrected. Patients should consume adequate amounts of calcium and vitamin D. If the patient does not receive enough calcium and/or vitamin D from food, supplements should be taken in the form of dietary supplements.
Gastrointestinal irritation
Oral bisphosphonates may cause local irritation of the upper gastrointestinal mucosa. In connection with these possible effects and the possibility of worsening the underlying disease, caution must be exercised when using the drug Ibandronic acid-Vista in patients with active diseases of the upper gastrointestinal tract (Barrett's esophagus, dysphagia, other diseases of the esophagus, gastritis, duodenitis, ulcers).
Adverse reactions such as esophagitis, esophageal ulcers, and esophageal erosions have been reported with the use of oral bisphosphonates, which in some cases were severe and required hospitalization, rarely with bleeding or subsequent development of stricture or perforation. The risk of severe esophageal adverse reactions is greater in patients who do not follow dosage recommendations and/or in individuals who continue to take oral bisphosphonates after symptoms suggestive of esophageal irritation have developed. Therefore, patients should strictly follow dosage recommendations (see section "Dosage and Administration").
Clinicians should be alert for any signs and symptoms indicating a possible esophageal reaction or esophageal irritation and inform patients to stop taking ibandronic acid and seek medical attention if dysphagia, pain with swallowing, chest pain, or the appearance of heartburn or worsening heartburn.
Although no increased risk was observed in controlled clinical studies, cases of gastric and duodenal ulcers have been reported with postmarketing use of oral bisphosphonates. Some of them were severe and had complications.
Acetylsalicylic acid and non-steroidal anti-inflammatory drugs (NSAIDs)
Since acetylsalicylic acid, NSAIDs and bisphosphonates can cause gastrointestinal irritation, caution should be exercised when using these drugs concomitantly with Ibandronic Acid-Vista.
Osteonecrosis of the jaw bones
Cases of osteonecrosis of the jaw bones have been reported with the use of bisphosphonates, usually observed during tooth extraction and/or in connection with local infections (including osteomyelitis) in patients with malignant neoplasms receiving treatment that included the administration of bisphosphonates.
Osteonecrosis of the jaw bone (ONJ) has been reported very rarely during post-marketing use in patients who received the drug for oncological indications (see Section “Adverse Reactions”).
Before starting treatment, patients with associated risk factors are recommended to undergo a dental examination with appropriate preventive intervention and an individual assessment of the benefit-risk ratio.
When assessing a patient's risk of osteonecrosis of the jaw bones, the following risk factors should be taken into account:
- the activity of the drug that inhibits bone resorption (risk higher for compounds with high activity), route of administration (risk higher with parenteral administration), and cumulative dose of bone resorption therapy;
- malignant neoplasms, concomitant pathological conditions (in particular, anemia, coagulopathy, infection), smoking;
- concomitant treatment: corticosteroids, chemotherapy, angiogenesis inhibitors, radiation therapy to the head and neck area;
- poor oral hygiene, periodontal disease, ill-fitting dentures, history of dental disease, invasive dental procedures such as tooth extraction.
During treatment, all patients should be advised to maintain good oral hygiene, undergo regular dental checkups, and promptly report any oral symptoms such as loose teeth, pain or swelling, or non-healing sores or discharge. During treatment, invasive dental procedures should only be undertaken after careful consideration and should be avoided in the immediate future following use of Ibandronic Acid-Vista.
The management plan for patients who develop osteonecrosis of the jaw bones should be developed in close collaboration between the physician and a dentist or oral surgeon experienced in the management of osteonecrosis of the jaw bones. The issue of interrupting treatment with Ibandronic acid-Vista for a period of three hours should be considered to improve the condition and, if possible, reduce associated risk factors.
Osteonecrosis of the external auditory canal
Osteonecrosis of the external auditory canal has been reported with bisphosphonates, mainly with long-term therapy. Possible risk factors for osteonecrosis of the external auditory canal include the use of steroid hormones and chemotherapy and/or local risk factors such as infection or trauma. The possibility of osteonecrosis of the external auditory canal should be considered in patients receiving bisphosphonates who have ear symptoms, including chronic ear infections.
Atypical hip fractures
Atypical femoral pedicle and shaft fractures have been reported during bisphosphonate treatment, primarily in patients receiving long-term treatment for osteoporosis. These transverse or subtransverse fractures can occur anywhere along the hip, from just below the lesser trochanter of the femur to just above the epicondyle. These fractures occur after minimal or no trauma, and some patients experience hip or groin pain, often associated with the characteristic features of a stress fracture, for several weeks to several months before the fracture manifests as a complete femur fracture . There are often two fractures, so the other hip should be examined in patients receiving bisphosphonate treatment who experience a femoral shaft fracture. Poor healing of these fractures has also been reported.
Discontinuation of bisphosphonates in patients with suspected atypical femoral fractures should be considered before completing the patient's assessment, taking into account an individual assessment of benefit and risk.
During treatment with bisphosphonates, patients should be advised to report hip, hip, or groin pain; All symptomatic patients should be evaluated for an incomplete femoral fracture.
Kidney failure
In clinical studies, there were no signs of renal dysfunction during long-term therapy with Ibandronic acid. However, during treatment with Ibandronic Acid-Vista, in accordance with the clinical assessment of each patient, it is recommended to monitor renal function, calcium, phosphorus and magnesium levels in the blood serum.
Rare hereditary problems
The drug contains lactose. Patients with rare hereditary problems such as galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take this drug.
Patients with hypersensitivity to other bisphosphonates
Caution should be exercised in patients with hypersensitivity to other bisphosphonates.
Disposal of unused and expired drugs
The release of the drug into the external environment must be minimized! The drug should not be disposed of in sewage or household waste. For disposal, it is necessary to use the so-called waste collection system, if available.
Use during pregnancy and breastfeeding
During preclinical studies, no signs of direct embryotoxic or teratogenic effects were found. The adverse effects of ibandronic acid in reproductive toxicity studies in animals were the same as for all bisphosphonates—decreased number of embryos, disruption of labor, increased incidence of visceral abnormalities (constriction of the ureteropelvic segment). There is no experience of clinical use in pregnant women.
FDA category of effect on the fetus is C.
Excreted in milk in animals. After 24 hours, the concentration of ibandronic acid in blood plasma and milk is the same and corresponds to 5% of the maximum. It is not known whether ibandronic acid is excreted in breast milk in women.
Side effects of the substance Ibandronic acid
With intravenous administration
From the nervous system and sensory organs: asthenia, headache, dizziness, insomnia, depression, uveitis, scleritis.
From the gastrointestinal tract: dyspeptic symptoms, diarrhea, constipation, gastritis, gastroenteritis.
From the musculoskeletal system: arthralgia, myalgia, pain in the limbs and bones, osteoarthritis.
From the respiratory system: bronchitis, upper respiratory tract infections, nasopharyngitis, bronchospasm in patients with aspirin-induced bronchial asthma.
From the genitourinary system: cystitis, urinary tract infections.
Other: fever, influenza-like syndrome, arterial hypertension, hypophosphatemia, decreased excretion of calcium by the kidneys, hypocalcemia, hypercholesterolemia, allergic reactions, reactions at the injection site, phlebitis, thrombophlebitis.
When taken orally
From the nervous system: dizziness, headache, weakness.
From the gastrointestinal tract: dyspepsia, nausea, vomiting, abdominal pain, dysphagia, flatulence, diarrhea, esophagitis, ulcer or stricture of the esophagus, gastroesophageal reflux, gastritis, duodenitis.
From the musculoskeletal system: myalgia, arthralgia, muscle stiffness, muscle spasm; in isolated cases - osteonecrosis of the jaw.
Allergic reactions: skin rash, urticaria, angioedema.
Other: hypocalcemia, decreased alkaline phosphatase activity, flu-like syndrome, back pain.
Interaction
Antacids and drugs containing polyvalent cations (for example, aluminum, magnesium, iron), products containing calcium, incl. milk and solid foods may interfere with the absorption of ibandronic acid (they should be consumed no earlier than 60 minutes after oral administration). Bisphosphonates and NSAIDs may cause irritation of the gastrointestinal mucosa (extra caution should be exercised when used concomitantly). Ranitidine (iv) increases the bioavailability of ibandronic acid by 20%. No dosage adjustment of ibandronic acid is required when used concomitantly with H2-antihistamines or other drugs that increase gastric pH. The solution for intravenous administration is incompatible with calcium-containing solutions and other solutions for intravenous administration.
Ibandronic acid does not affect the activity of the main isoenzymes of the cytochrome P450 system. At therapeutic concentrations, it weakly binds to plasma proteins, so it is unlikely that it will displace other drugs from protein binding sites.
Buy Bonviva film-coated tablets 150 mg No. 1 in pharmacies
Bonviva Buy Bonviva in pharmacies DOSAGE FORMS film- coated tablets 150mg film-coated tablets 150mg
MANUFACTURERS Hoffmann-La Roche Ltd (Switzerland) Hoffmann-La Roche Ltd packaged Rainbow Production (Switzerland)
GROUP Antiosteoporotic agents - biosphosphonates
COMPOSITION Active ingredient: ibandronic acid.
INTERNATIONAL NON-PROPENTED NAME Ibandronic acid
SYNONYMS Bondronate
PHARMACOLOGICAL ACTION Ibandronic acid is a highly active nitrogen-containing bisphosphonate, an inhibitor of bone resorption and osteoclast activity. Ibandronic acid prevents bone destruction caused by gonadal suppression, retinoids, tumors and tumor extracts in vivo. In studies in young (fast-growing) rats, ibandronic acid also inhibited endogenous bone resorption, resulting in increased bone mass compared to control animals. In animal models, ibandronic acid has been confirmed to be a potent inhibitor of osteoclast activity and does not impair bone mineralization even when administered at doses more than 5000 times higher than doses for the treatment of osteoporosis. With long-term use of ibandronic acid in two different dosing regimens (daily or intermittent dosing with an extended period without treatment), new normal bone formation and/or an increase in mechanical strength was observed in studies in rats, dogs and monkeys, even at doses above therapeutic levels. including doses in the toxic range. The effectiveness of the drug in both regimens was confirmed in the clinical study MF4411 - daily administration of 2.5 mg or intermittent administration of 20 mg of the drug over a period of 9-10 weeks without treatment led to a decrease in the incidence of fractures. In post-monopausal women, oral administration of the drug (both daily and intermittent administration of the drug with a period of 9-10 weeks without treatment) led to biochemical changes characteristic of dose-dependent inhibition of bone resorption, including a decrease in the concentration of biochemical markers of bone collagen breakdown (deoxypyridinoline and cross-linked C- and N-telopeptides of type I collagen) in urine. After cessation of treatment, there is a return to the previous, pre-treatment, increased level of bone resorption, characteristic of postmenopausal osteoporosis. Histological analysis of bone biopsies taken from post-menopausal women in the second and third years of treatment showed the presence of normal bone tissue, as well as the absence of mineralization defects. In a phase 1 bioequivalence study involving 72 postmenopausal women, subjects received the drug orally every 28 days (4 doses total). In this study, it was found that a decrease in serum cross-linked C-telopeptide type 1 collagen (CTX) concentrations was observed as early as the first 24 hours after the first dose (an average of 28%), and an average maximum decrease in concentration (of 69%). ) was observed after 6 days. After the 3rd and 4th doses, the mean maximum decrease in concentration 6 days after each dose was 74%, and 28 days after the 4th dose, the average decrease in concentration was 56%. When the drug was stopped after the 4th dose, the concentration of biochemical markers showed the cessation of the inhibitory effect of the drug on bone resorption. Ibandronic acid does not affect the process of replenishment of the osteoclast pool. The selective effect of ibandroic acid on bone tissue is due to its high affinity for hydroxyapatite, which constitutes the mineral matrix of bone. Ibandronic acid inhibits bone resorption and does not have a direct effect on bone formation. In postmenopausal women, it reduces the increased rate of bone turnover to reproductive age levels, which leads to a progressive increase in bone mass. Daily or intermittent administration of ibandroic acid results in a decrease in bone resorption, as demonstrated by decreased concentrations of biochemical markers of bone turnover in urine and serum, an increase in bone mineral density (BMD), and a decrease in the incidence of fractures. The high activity and breadth of the therapeutic range provide the possibility of a flexible dosage regimen in relatively low doses and intermittent use of the drug with a long period without treatment. Efficiency. Bone mineral density (BMD). In a 2-year, double-blind, multicenter study (BM16549) with the participation of postmenopausal women suffering from osteoporosis (BMD of the lumbar vertebrae: initial T-criterion below -2.5 SD), based on an increase in BMD, it was shown that prescribing the drug once a month characterized by at least the same effectiveness as taking the drug at a dose of 2.5 mg daily. Data obtained in the primary analysis after the first year of the study were confirmed in the subsequent analysis after the second year of the study. In addition, a prospective analysis showed that the drug at a dosing regimen of 150 mg once a month is superior to the drug 2.5 mg daily in terms of the increase in lumbar vertebrae BMD After the first year of the study (primary analysis) in 91.3% (p = 0.005) of patients receiving the drug once a month, compared with 84.0% of patients receiving the drug 2.5 mg daily, there was an increase in lumbar vertebrae BMD or maintenance of its original level. By the end of the second year, 93.5% (rIZ.004) of patients receiving the drug 150 mg once a month and 86.4% of patients receiving the drug 2.5 mg daily had a positive response to therapy. Regarding hip BMD values, after the first year of the study, 90.0% (p less than 0.001) of patients receiving the drug 150 mg once a month and 76.7% of patients receiving the drug 2.5 mg daily experienced an increase in BMD or maintained its original level. By the end of the second year, 93.4% (p less than 0.001) of patients receiving the drug 150 mg once a month and 78.4% of patients receiving the drug 2.5 mg daily had an increase in hip BMD or maintained its original level. Using a more stringent criterion that included global assessment of lumbar vertebrae and hip BMD, at the end of the first year of the study, a positive response was observed in 83.9% (p less than 0.001) of patients receiving the drug 150 mg once a month, and in 65.7% of patients who received the drug 2.5 mg daily. By the end of the second year, in 87.1% (p less than 0.001) of patients receiving Bonviva 150 mg once a month, and in 70.5% of patients receiving 2.5 mg daily. Biochemical markers of bone resorption. Clinically significant reductions in serum CTX concentrations were obtained after 3, 6, 12 and 24 months of therapy. After a year of treatment with the drug 150 mg once a month (primary analysis), the average reduction was 76%, and when taking the drug at a dose of 2.5 mg daily - 67%. By the end of the second year of the study, the average reduction was 68% when taking the drug 150 mg once a month, and 62% when taking the drug at a dose of 2.5 mg daily. A decrease in CTX concentration of more than 50% compared to the initial value was observed in 83.5% (p = 0.006) of patients receiving the drug 150 MG once a month, and in 73.9% of patients receiving the drug 2.5 mg daily, during the first year of the study. By the end of the second year, a positive response to therapy was observed in 78.7% (p-0.002) of patients receiving the drug 150 mg once a month, and in 65.6% of patients receiving the drug 2.5 mg daily. The BM16549 study showed that 150 mg once monthly and 2.5 mg daily were at least equally effective in reducing the risk of fractures. Preclinical safety data. In animal studies, the toxic effect was observed only at drug exposures significantly greater than the maximum drug exposure in humans, and therefore appears to be of little significance for the clinical use of the drug. No data indicating possible carcinogenic and genotoxic activity have been identified. Pharmacokinetics. There was no direct relationship between the effectiveness of ibandroic acid and the concentration of the substance in the blood plasma. Similar effectiveness of ibandroic acid when taken in different dosing regimens (daily or at intervals of several weeks) has been shown in various studies conducted in both human volunteers and animal studies. The total dose received throughout the study period was identical. In rats, the interval between doses of the drug with an intermittent dosing regimen was at least 6 weeks, in dogs 11 weeks, in monkeys 30 days and in humans 9.5 weeks. Suction. After oral administration, ibandronic acid is rapidly absorbed from the upper gastrointestinal tract. Plasma concentrations increase dose-dependently when the dose is increased to 50 mg and significantly more when the dose is further increased. The time to reach maximum concentration is 0.5-2 hours (median - 1 hour) after administration on an empty stomach, absolute bioavailability is 0.6%. Concomitant consumption of food or drinks (except pure water) reduces the bioavailability of ibandroic acid by 90%. When taking ibandroic acid 60 minutes before meals, no significant decrease in bioavailability is observed. Ingestion of food or liquid less than 60 minutes after ibandroic acid reduces its bioavailability and the resulting increase in MIC. Distribution. After entering the systemic circulation, ibandronic acid quickly binds to bone tissue or is excreted in the urine. 40-50% of the amount of the drug circulating in the blood penetrates the bone tissue and accumulates in it. Apparent final volume of distribution 90 l. The binding to plasma proteins at therapeutic concentrations is quite low (about 85%), thus, the likelihood of drug-drug interactions due to displacement from binding to plasma proteins is small. Metabolism. There is no evidence that ibandronic acid is metabolized in animals or humans. Excretion. 40-50% of the oral dose absorbed into the bloodstream is bound in the bones, and the rest is excreted unchanged by the kidneys. The drug that is not absorbed is excreted unchanged in the feces. The observed apparent terminal half-life varies widely (10-72 hours) and depends on the dose of the drug and the sensitivity of the assay. The concentration of the drug in the blood plasma decreases quickly and is 10% of the maximum 3 and 8 hours after intravenous administration and oral administration, respectively. The total clearance of ibandroic acid is low, its average values are in the range of 84-160 ml/min. Renal clearance (60 ml/min. in healthy postmenopausal women) accounts for 50-60% of the total clearance and depends on creatinine clearance. The difference between total and renal clearance reflects the uptake of the substance into bone tissue. Pharmacokinetics in special groups of patients. The pharmacokinetics of ibandroic acid does not depend on gender. There were no clinically significant interracial differences in the distribution of ibandronic acid in individuals of the Caucasian and Mongoloid races. There is not enough data regarding the Negroid race. Patients with impaired renal function. In patients with impaired renal function, the renal clearance of ibandroic acid depends linearly on creatinine clearance (CC). For patients with mild or moderate renal impairment (creatinine clearance>30 ml/min), no dose adjustment is required, according to the results of the BM 16549 study, where the majority of patients had impaired renal function. In patients with severe renal impairment (creatinine clearance <30 ml/min) who received the drug at a dose of 10 mg orally for 21 days, the concentration of ibandroic acid in the blood plasma was 2-3 times higher than in people with normal renal function ( total clearance 129 ml/min). In severe renal impairment, the total clearance of ibandroic acid is reduced to 44 ml/min. However, an increase in systemic concentration does not impair the tolerability of the drug. Patients with impaired liver function. There are no data on the pharmacokinetics of ibandroic acid in patients with impaired liver function. The liver does not play a significant role in the clearance of ibandroic acid, which is not metabolized but is excreted through the kidneys and by binding to bone tissue. Therefore, for patients with impaired liver function, no dose adjustment is required. Since at therapeutic concentrations ibandronic acid binds weakly to plasma proteins (85%), it is likely that hypoproteinemia in severe liver disease does not lead to a clinically significant increase in the concentration of the free substance in the blood. Elderly age. The studied pharmacokinetic parameters do not depend on age (multivariate analysis). The possible decrease in renal function in elderly patients should be taken into account. Children. There are no data on the use of the drug in persons under 18 years of age.
INDICATIONS FOR USE Postmenopausal osteoporosis to prevent fractures.
CONTRAINDICATIONS Hypersensitivity to ibandronic acid or other components of the drug. Hypocalcemia. Before starting the drug, as with all bisphosphonates used to treat osteoporosis, hypocalcemia should be eliminated. As with other bisphosphonates, contraindications include lesions of the esophagus that delay its emptying, such as stricture or achalasia. Inability to sit or stand for 60 minutes. Hereditary galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption. Severe renal impairment (creatinine clearance <30 ml/min).
SIDE EFFECTS The most common adverse reactions were arthralgia and flu-like syndrome, which were usually observed after taking the first dose of the drug, were characterized by a weak or moderate degree of intensity, short duration and resolved without treatment. The safety of ibandronic acid (2.5 mg daily) was assessed in four placebo-controlled clinical trials (N=1251). The majority of patients participating in these studies had previously participated in the main 3-year study of MF4411. The overall safety profile of ibandronic acid (2.5 mg daily) in all of the above studies was similar to that of placebo. In the 2-year study BM 16549 in postmenopausal women with osteoporosis, the overall safety profile of 150 mg once monthly was similar to that of 2.5 mg daily. The overall proportion of patients experiencing adverse reactions was 22.7% and 25.0% after 1 year and 2 years of dosing 150 mg once monthly, respectively. In most cases, adverse reactions were mild or moderate in intensity and did not lead to discontinuation of the drug. Adverse reactions that have a cause-and-effect relationship with taking the drug (according to researchers) are distributed according to classes of organ systems. From the immune system. Rarely: hypersensitivity reactions; very rare: anaphylactic reactions/shock, allergic reactions, in particular exacerbation of bronchial asthma. From the nervous system. Often: headache; uncommon: dizziness. From the side of the organ of vision. Rare: inflammatory eye diseases. From the gastrointestinal tract. Common: esophagitis, gastritis, gastroesophageal reflux disease, dyspepsia, diarrhea, abdominal pain, nausea; uncommon: esophagitis, including esophageal ulceration or stricture, dysphagia, vomiting, flatulence; rarely: duodenitis. From the skin and subcutaneous fat. Common: rash; rarely: angioedema, facial swelling, urticaria. From the musculoskeletal system. Common: arthralgia, myalgia, musculoskeletal pain, muscle spasms, musculoskeletal stiffness; uncommon: back pain; rarely: atypical subtrochanteric and diaphyseal fractures of the femur; very rare: osteonecrosis of the jaw. From the body as a whole. Often: influenza-like syndrome; uncommon: fatigue. Description of individual adverse reactions. Adverse reactions from the gastrointestinal tract. In clinical trials of patients with a history of gastrointestinal disease, including peptic ulcer disease, without recent bleeding or hospitalization, and patients with dyspepsia or reflux receiving appropriate therapy, there was no difference in the incidence of upper adverse events. gastrointestinal tract when taking the drug in various dosage regimens (2.5 mg daily and 150 mg once a month). In the BM16549 study, after the first and second year of taking the drug, there were no differences in laboratory parameters in both groups with different dosing regimens (2.5 mg daily and 150 mg once a month). Flu-like syndrome. Influenza-like syndrome may include acute phase reactions or symptoms such as myalgia, arthralgia, fever, chills, fatigue, nausea, loss of appetite, or bone pain. Osteonecrosis of the jaw. Most cases of osteonecrosis of the jaw that developed with the use of bisphosphonates were reported in cancer patients, with a few cases in patients with osteoporosis. Osteonecrosis of the jaw has mainly been associated with tooth extraction and/or local infection (particularly osteomyelitis). Other risk factors for the development of osteonecrosis of the jaw include an established diagnosis of cancer, chemotherapy, radiation therapy, the use of glucocorticosteroids and insufficient oral hygiene. Inflammatory eye diseases. Inflammatory ocular diseases such as episcleritis, scleritis and uveitis have been reported during therapy with bisphosphonates, including ibandronic acid. In some cases, despite treatment, recovery occurred only after discontinuation of bisphosphonates. Anaphylactic reactions/shock. Cases of anaphylactic reactions/shock, including death, have been reported during treatment with intravenous ibandronic acid.
INTERACTIONS Products containing calcium and other polyvalent cations (for example, aluminum, magnesium, iron), including milk and solid foods, may interfere with the absorption of the drug (which is consistent with data from animal studies), they should be consumed no earlier than 60 minutes after oral administration of the drug. Calcium supplements, antacids, and oral medications containing polyvalent cations (eg, aluminum, magnesium, iron) may interfere with the absorption of ibandronic acid and should therefore be taken no earlier than 60 minutes after dosing. Pharmacokinetic studies in postmenopausal women showed the absence of any drug-drug interaction between ibandronic acid and tamoxifen or hormone replacement therapy (estrogen). There was also no evidence of drug-drug interaction with the simultaneous use of ibandronic acid and melphalan/prednisolone in patients with multiple myeloma. Bisphosphonates and nonsteroidal anti-inflammatory drugs (NSAIDs) may cause GI irritation. Particular caution should be exercised when using NSAIDs concomitantly with the drug. In a clinical study of postmenopausal women with osteoporosis (BM16549), concomitant use of acetylsalicylic acid or other NSAIDs and the drug (2.5 mg daily or 150 mg once monthly) for 1 year resulted in similar rates of upper gastrointestinal side effects. In studies involving healthy volunteers (men) and postmenopausal women, IV ranitidine increased the bioavailability of ibandronic acid by 20%, probably due to a decrease in gastric acidity. However, this increase is within the normal bioavailability limits of ibandronic acid. No dosage adjustment of ibandronic acid is required when used concomitantly with H2-histamine receptor blockers or other drugs that increase gastric pH. Since ibandronic acid does not inhibit the main isoenzymes of the cytochrome P450 system, and studies in rats have shown the absence of its inducing effect, the presence of clinically significant drug-drug interactions is unlikely. At therapeutic concentrations, ibandronic acid binds weakly to plasma proteins and is therefore unlikely to displace other drugs from protein binding sites. Ibandronic acid is excreted only through the kidneys and does not undergo any biotransformation. It appears that the elimination pathway of ibandroic acid does not involve any of the transport systems involved in the elimination of other drugs. Study BM16549, involving 1,500 patients, compared dosing regimens of ibandronic acid (daily versus monthly); of these, 14% of subjects were also taking H2-receptor blockers or proton pump inhibitors. The frequency of adverse events from the upper gastrointestinal tract was the same for different dosing regimens (150 mg once a month and 2.5 mg daily).
DOSAGE AND ADMINISTRATION Orally, 150 mg (1 tablet) once a month (preferably on the same day of each month), 60 minutes before the first meal of the day, liquid (except water) or other medicines and food supplements (including calcium). The tablets should be swallowed whole with a glass (180-240 ml) of clean water while sitting or standing and do not lie down for 60 minutes after taking the drug. The tablets should not be chewed or sucked due to possible ulceration of the upper gastrointestinal tract. Do not use mineral waters that contain a lot of calcium. If you miss a scheduled dose, you should take one tablet of the drug 150 mg, if there are more than 7 days before the scheduled dose, and then take the drug once a month in accordance with the established schedule. If there are less than 7 days before the next scheduled appointment, you must wait until the next scheduled appointment, and then continue taking it in accordance with the established schedule, because You should not take more than 1 tablet of the drug per week. Dosing in special patient groups. Liver dysfunction. No dose adjustment is required. Renal dysfunction. For mild and moderately severe renal dysfunction (creatinine clearance >30 ml/min), no dose adjustment is required. When creatinine clearance is <30 ml/min, the use of the drug is not recommended, since clinical experience is limited. Elderly age. No dose adjustment is required. Safety and effectiveness in persons under 18 years of age have not been established.
OVERDOSE Possible symptoms when taken orally. Adverse events from the upper gastrointestinal tract, such as indigestion, heartburn, esophagitis, gastritis, upper gastrointestinal ulcer. Treatment. There is no information on treatment in case of drug overdose. Milk or antacids are used to bind the drug. Due to the risk of irritation of the esophagus, vomiting should not be induced and the patient should remain in an upright standing position.
SPECIAL INSTRUCTIONS Use caution. Active pathological processes localized in the upper gastrointestinal tract (for example, established Barrett's esophagus, dysphagia, other diseases of the esophagus, gastritis, duodenitis or ulcers). Pregnancy and lactation period. Pregnancy. The drug should not be used during pregnancy. There was no evidence of direct embryotoxic or teratogenic effects in rats and rabbits treated with ibandronic acid orally; no adverse effects on offspring development were found in F1 rats. The adverse effects of ibandronic acid in reproductive toxicity studies in rats were the same as for all bisphosphonates - decreased embryo production, disruption of labor (dystocia), increased incidence of visceral abnormalities (constriction of the ureteropelvic segment). No specific studies have been conducted on once-a-month dosing regimens. There is no experience of clinical use of the drug in pregnant women. Breastfeeding period. Excreted in milk in rats. In lactating rats with intravenous administration of ibandronate in doses of 0.08 mg/kg per day, the highest concentration of ibandronic acid in breast milk was observed in the first 2 hours after intravenous administration and amounted to 8.1 ng/ml. After 24 hours, the concentration of ibandroic acid in blood plasma and milk was the same and corresponded to 5% of the maximum. It is not known whether ibandronic acid is excreted in breast milk in women. The drug should not be used during breastfeeding. Osteoporosis can be confirmed by detecting low BMD (T index < -2.0 SD (Standard deviation) and a fracture (including a history) or low bone mineral density (T index < -2.5 SD) in the absence of confirmed fracture Hypocalcemia Before using the drug, hypocalcemia and other disorders of bone metabolism and electrolyte balance should be corrected Patients should consume sufficient amounts of calcium and vitamin D. If the patient does not receive enough calcium and vitamin D from food, then they should be additionally taken in the form of dietary supplements Irritation of the gastrointestinal tract. The use of oral bisphosphonates can lead to local irritation of the mucous membrane of the upper gastrointestinal tract. Due to the possible irritant effect of the drug and the worsening of the course of an existing underlying gastrointestinal disease, caution should be exercised when prescribing the drug to patients with active pathological processes , localized in the upper gastrointestinal tract (for example, established Barrett's esophagus, dysphagia, other diseases of the esophagus, gastritis, duodenitis or ulcers). In patients receiving treatment with oral bisphosphonates, cases of adverse events such as esophagitis, ulcers or erosions of the esophagus, rarely accompanied by bleeding or the development of further strictures or perforations of the esophagus, have been described. In some cases, adverse events were severe and required hospitalization. The risk of severe esophageal adverse events appears to be greater in patients who do not adhere to dosage regimens and/or who continue to take oral bisphosphonates after the onset of symptoms suggestive of esophageal irritation. Patients should carefully read the recommendations for taking the drug and carefully follow them. Clinicians should be especially alert for any signs or symptoms indicating a possible esophageal reaction, and patients should be warned to stop taking the drug and seek medical attention if they experience dysphagia, pain when swallowing or behind the sternum, new or worsening heartburn. When using oral bisphosphonates (post-registration surveillance), isolated cases of the development of gastric and duodenal ulcers, sometimes severe and complicated, have been described, although in clinical studies no increase in the risk of these diseases was observed. Since the use of NSAIDs and bisphosphonates may be accompanied by irritation of the mucous membrane of the gastrointestinal tract, caution should be exercised when using NSAIDs simultaneously with the drug. Osteonecrosis of the jaw. Cases of osteonecrosis of the jaw have been reported with the use of bisphosphonates. Most cases have been reported in cancer patients during dental procedures, with a few cases in patients with postmenopausal osteoporosis or other diseases. Risk factors for the development of osteonecrosis of the jaw include an established diagnosis of cancer, concomitant therapy (chemotherapy, radiation therapy, corticosteroids) and other disorders (anemia, coagulopathy, infection, existing dental disease). Most reported cases have occurred with intravenous bisphosphonates, but isolated cases have occurred in patients receiving oral medications. Dental surgery during bisphosphonate therapy can increase the manifestations of osteonecrosis of the jaw. It is unknown whether discontinuation of bisphosphonates reduces the risk of osteonecrosis. The decision to conduct treatment must be made for each patient individually after assessing the risk/benefit ratio. Atypical hip fractures. Atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving bisphosphonates, primarily in patients receiving long-term treatment for osteoporosis. Transverse and short oblique fractures can be localized along the entire length of the femur from the lesser trochanter to the supracondylar eminence. Atypical fractures occur spontaneously or as a result of minor injuries. In the weeks or months before a hip fracture occurs, some patients experience pain in the hip or groin area, which is often accompanied by radiographic evidence of a stress fracture. Because atypical fractures are often bilateral, it is necessary to monitor the other hip in patients with a femoral shaft fracture. Poor healing of atypical fractures was noted. If an atypical fracture is suspected and pending examination results, discontinuation of bisphosphonate therapy should be considered based on an assessment of the benefit/risk ratio in each individual case. Patients should be advised to report any hip or groin pain during bisphosphonate therapy. If these symptoms are present, it is necessary to conduct an examination to identify an incomplete hip fracture.
STORAGE CONDITIONS Store in a dry place, out of reach of children, at a temperature not exceeding 30 C
Directions for use and doses
Inside, intravenously. Orally, 60 minutes before the first meal of the day, liquid (except water) or other drugs and food additives, swallow whole with a glass (180-240 ml) of clean water, in the “sitting” or “standing” position (not you should lie down within 60 minutes after taking the drug). The tablets should not be chewed or sucked due to possible ulceration of the upper gastrointestinal tract. Do not use mineral water, which contains a lot of calcium. Tablets 2.5 mg: 1 tablet. 1 time per day. Tablets 150 mg: 1 tablet. Once a month (preferably on the same day of each month). If you miss a scheduled appointment, you should take 1 tablet. 150 mg, if there are more than 7 days before the scheduled dose, and then take the drug once a month in accordance with the established schedule. If there are less than 7 days before the next scheduled appointment, you must wait until the next scheduled appointment and then continue taking it in accordance with the established schedule, because You cannot take more than 1 tablet. in Week. Tablets 50 mg: for metastatic bone lesions - 50 mg 1 time per day.
IV is used only in a hospital setting. Before use, the contents of the ampoule are diluted with the required amount of isotonic sodium chloride solution or 5% dextrose solution. The dose is determined individually and depends on the severity of hypercalcemia and the type of tumor. In patients with severe calcemia (serum calcium adjusted for serum albumin ≥3 mmol/l) - a single dose of 4 mg. For moderate calcemia (<3 mmol/l) - 2 mg once. The maximum single dose - 6 mg - does not increase the effect. If the first administration is insufficiently effective or if hypercalcemia recurs, repeated administration is possible.
Serum albumin-corrected calcium concentration (mmol/L) is calculated as follows: serum calcium (mmol/L) – [0.02 x albumin (g/L)] + 0.8 or in mg/dL: serum calcium (mg /dl) + 0.8 × [4 − albumin (g/dl)].
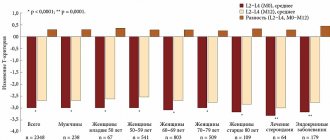
Purpose of the study. To evaluate the effectiveness of intravenous ibandronic acid on bone mineral density (BMD) in women with osteoporosis.
Material and methods. 30 women with osteoporosis aged 50-80 years (average age 62.7±9.0 years) were examined. The diagnosis of osteoporosis was verified based on the T-criterion in the study areas, according to WHO criteria. The absolute values of BMD of the lumbar spine (L—L!V), proximal femur and femoral neck were determined before treatment and 12 months after it. Ibandronic acid for intravenous administration was administered as a bolus over 30 s at a dose of 3 mg once every 3 months.
To process the measurement results, statistical analysis methods were used (SPSS Statistics 20.0), Wilcoxon and Mann-Whitney tests were used.
Results. Ibandronic acid for intravenous administration has a positive effect on changes in absolute BMD in the lumbar spine and proximal femur, including the femoral neck, causing their increase 12 months after treatment.
Conclusion. Intravenous ibandronic acid is an effective means of increasing BMD in patients with osteoporosis.
Osteoporosis is a chronic progressive disease; it is most common in the female population during menopause [1]. Given the constant increase in the proportion of women over 50 years of age in the general population of European countries, the treatment of osteoporosis and the need for mandatory drug prevention of fractures creates a significant financial burden on the healthcare industry [2]. Currently, the main group of drugs used in the treatment of osteoporosis are oral bisphosphonates, which have proven effective in preventing fractures [3]. However, the prescription of these drugs may be limited due to the presence of contraindications from the gastrointestinal tract or the inability to take them in patients with prescribed bed rest or in the presence of a conflict with other vital drugs. Another significant problem with the use of oral bisphosphonates is low compliance and adherence to treatment. According to studies, after 6 months, every 5th patient violates the prescribed regimen for taking tablet bisphosphonates, and by the end of 1 year, every 2nd patient independently stops oral therapy [4]. Therefore, an alternative is the active introduction of bisphosphonates for intravenous administration into clinical practice.
The purpose of this study was to evaluate the effectiveness of intravenous ibandronic acid on bone mineral density (BMD) in women with osteoporosis.
Material and methods
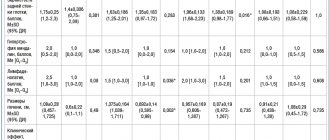
The study included women over 50 years of age with instrumentally verified osteoporosis with or without a history of fractures. Treatment with the drug ibandronic acid for intravenous administration (Bonviva®) was carried out according to the indications for antiresorptive therapy in the metabolic disorders room of the Republican Scientific and Practical Center for Radiation Medicine and Human Ecology (Gomel). Using cubital vein venipuncture, the drug was administered in a stream over 30 s at a dose of 3 mg once every 3 months. Before the therapy, the patients were advised to drink plenty of fluids the day before, as well as daily intake of calcium (at least 1000 mg) and vitamin D (400 to 800 IU) throughout the observation period. Previous use of other antiresorptive drugs was not a criterion for excluding them from the study. The end point for assessing the effectiveness of treatment was the study of the absolute values of BMD of the lumbar spine, proximal femur (POF) and femoral neck (FNC) before the start of therapy and at least 12 months after 4 intravenous injections of ibandronic acid. A reliable criterion for the increase in MIC was exceeding the minimum significant error threshold, which is 1.5%. Instrumental measurement of BMD was performed using dual-energy X-ray absorptiometry (DXA) of the lumbar vertebrae (L,—L|V), FB and SBK (LUNAR Prodigy, (USA) with CORE v 8.5 software). The measurement results are presented in absolute values: in g/cm2 for the lumbar spine and mg/cm2 for the POB.
The diagnosis of osteoporosis was made according to WHO criteria when the T-criterion value in one of the study zones was -2.5 or less. The values of the BMD of the PHB and BBC are presented as the average values of the study on both sides for each patient. In the case of a prosthetic hip joint, measurements were performed on the intact limb [5]. The survey of study participants was carried out using a unified computer program developed at the Republican Scientific and Practical Center for Radiation Medicine and Human Ecology and registered with the NCIS (No. 116 of December 15, 2009, G. N. Romanov, L. S. Starostenko, E. V. Rudenko). The questionnaire included sections about previously suffered low-traumatic fractures, family history of osteoporotic fractures in first-degree relatives aged over SO years, age at menopause, as well as the presence of diseases associated with the development of osteoporosis. The questionnaire took into account information about previous use of calcium supplements in combination with vitamin D and anti-osteoporotic therapy.
Statistical data processing was carried out using the SPSS Statistics 2O.O computer program. Methods of parametric and nonparametric statistics were used for analysis. Data are presented in the format “mean (+95% CI; -95% CI)” or “mean±standard deviation”. To determine the statistical significance of differences in dependent samples, the Wilcoxon test or t-test for paired samples was used; in independent samples, the Mann-Whitney test was used. The dynamics of changes in BMD were assessed by the level of increase in density indicators, expressed as a percentage of the initial level. Changes in indicators over time were defined as statistically significant at P
L I T E R A T U R A 1. Siris E. S. //JAMA.—2001.—Vol. 286,— P. 2815—2822. 2. Melton LJ // J. Bone Miner. Res.—2003.—Vol. 18.— P. 1139—1141. 3. Chesnut S. H. // J. Bone Miner. Res.—2004.—Vol. 19.— P. 1241—1249. 4. Official Internet portal of the International Osteoporosis Foundation // [Electronic resource] / International Osteoporosis Foundation. - Nyon, 2014. - Access mode: https:// www.iofbonehealth.o rg/s ites/defa ult/fil es/PD Fs/s tayin g_ power_2006.pdf. 5. Lewiecki EM // Bone.— 2008.— Vol. 43.— P. 1115—1121. 6. Miller PD // Clin. Exp. Rheumatol.—2008.—Vol. 26.— P. 1125—1133. 7. Miller PD //Bone.—2011.—Vol. 49.— P. 1317—1322. 8. Filleul O., Crompot E., Saussez S. // J. Cancer Res. Clin. Oncol.—2010.—Vol. 136.— P. 1117—1124. 9. Briot K. // Joint Bone Spine. - 2012. - Vol. 79.— P. 304—313. 10. Eriksen EF // J. Bone Miner. Res.—2009.—Vol. 24.— P. 1308—1313.
Received 09.17.14.
Address for correspondence: Romanov Georgy Nikitich.
Gomel State Medical University. 246050, Gomel, st. Lange, 5; sl. tel. (8-0232) 49-19-62. The article was prepared with the participation of Roche Products Limited. Key words:
women, ibandronic acid, bone mineral density, osteoporosis
Author(s):
Romanov G. N., Chernova N. F.
Medical institution: Gomel State Medical University, Republican Scientific and Practical Center for Radiation Medicine and Human Ecology of the Ministry of Health of the Republic of Belarus