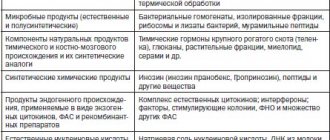
Inosine pranobex
Inosine pranobex is taken orally at regular intervals (8 or 6 hours) 3-4 times a day. The drug is taken after meals with a small amount of water.
Recommended doses and dosage regimens:
Adults
: 50 mg/kg body weight per day (in terms of the required number of tablets), divided into 3-4 doses;
Children aged 3 to 12 years:
50 mg/kg body weight per day (in terms of the required number of tablets), divided into 3-4 doses.
In severe forms of infectious diseases, the dose can be increased individually to 100 mg/kg body weight per day, divided into 4-6 doses.
The maximum daily dose for adults is 3-4 g/day, for children aged 3 years and older - 50 mg/kg/day.
For acute diseases
Treatment usually lasts from 5 to 14 days. After the symptoms disappear, treatment should be continued for 1 to 2 days or more, depending on the indications.
For chronic recurrent diseases
Treatment in adults and children is carried out in courses lasting 5-10 days at intervals of 8 days. The duration of maintenance treatment can be up to 30 days, and the dose can be reduced to 500 - 1000 mg/day.
For herpetic infection, adults and children are prescribed for 5-10 days until the symptoms of the disease disappear; in the asymptomatic period, adults are recommended to undergo maintenance treatment of 500 mg 2 times a day for 30 days to reduce the number of relapses.
For papillomavirus infection
For adults, the drug is prescribed 1000 mg 3 times a day (in terms of the required number of tablets), for children 50 mg/kg body weight per day (in terms of the required number of tablets), divided into 3-4 doses, for 14-28 days. as monotherapy.
For cervical dysplasia
, associated with the human papillomavirus, adults are prescribed the drug 1000 mg 3 times a day (in terms of the required number of tablets) for 10 days, then 2-3 similar courses are administered with an interval of 10-14 days.
For recurrent genital warts
For adults, the drug is prescribed 1000 mg 3 times a day (in terms of the required number of tablets), for children 50 mg/kg body weight per day (in terms of the required number of tablets), divided into 3-4 doses, either as monotherapy or in combinations with surgical treatment for 14-28 days, then a three-time course.
Use in elderly patients
There is no need for dose adjustment; the drug is used in the same way as in middle-aged patients. Elderly patients are more likely to experience increased concentrations of uric acid in serum and urine than middle-aged patients.
Use in children
Inosine pranobex is used in children over 3 years of age.
Use in patients with renal and hepatic insufficiency
During treatment with the drug, the level of uric acid in the blood serum and urine should be monitored every 2 weeks. Monitoring the activity of liver enzymes is recommended every 4 weeks during long courses of treatment with the drug.”
Introduction
The issues of antiviral therapy for respiratory infections in children are currently extremely relevant.
The pandemic of the new coronavirus infection caused by the SARS-Cov-2 virus has once again demonstrated that the use of drugs with antiviral effects in the first 48 hours from the onset of clinical manifestations of the infection reduces the severity of the disease and improves the outcome of the disease. The prescription of antiviral therapy for respiratory infections in children is determined by the patient’s age, the severity of the condition and the etiology of the disease. In addition to a large group of respiratory viruses, the cause of acute respiratory infections, including recurrent ones, can be viruses of the herpes group.
The role of active forms of herpesvirus infections (HVI) in the recurrence of respiratory tract infections in children and the formation of chronic foci of infection in the adenotonsillar zone has been shown. According to A.S. Levina et al., recurrent respiratory diseases in children from 1 year to 6 years are associated with GVI in 75% of cases [1]. The most frequently detected active forms of infection are those caused by human herpes virus (HHV) 6A/B, and somewhat less frequently by Epstein-Barr virus (EBV) and cytomegalovirus (CMV) [2]. Previous studies have shown that in a group of patients with monthly respiratory diseases, markers of HVI are detected in 91% of cases. Moreover, according to our research, in the group of children with monthly respiratory infections, the proportion of active forms is about 40%, which once again emphasizes the need for an integrated approach to diagnosing the etiology of recurrent diseases of the respiratory tract [3].
In recent years, the problem of combined GVI has become relevant. The presence of several herpesviruses in active form at once in the acute phase of the disease changes the clinical picture and complicates laboratory diagnosis of the condition.
In the work of N.G. Yaroslavtseva [4] demonstrated that the concentration of HHV-6 DNA in peripheral blood leukocytes and plasma of adult patients is almost 3 times lower in mixed HVI than in HHV-6 monoinfection (5.9 × 104 and 2 × 104, respectively). Earlier studies demonstrated the ability of HHV-6 to activate EBV replication from a latent state during co-infection [5, 6]. In turn, the presence of the EBV genome made B cells more susceptible to HHV-6 infection [7]. The combination of HHV-6 and EBV infection made it difficult to diagnose active forms of HVI in pregnant women [8] and in children after liver and kidney transplantation [9].
One of the most studied antiviral agents worldwide is inosine pranobex [10, 11]. The drug can affect the levels of viral RNA and is active against herpes simplex virus (HSV), human papillomavirus, human immunodeficiency virus, influenza viruses and acute respiratory infections, CMV infection and EBV [12].
Inosine pranobex (a synthetic compound of para-aminobenzoate NN-dimethylamino-2-propanol with inosine in a 3:1 molar ratio) has been widely used since 1971 as an antiviral agent, affects the immune system, enhancing the proliferation of T-lymphocytes and the activity of natural killer cells, increasing cytokine levels [12–15].
Currently, the antiviral effects of inosine pranobex are explained by three mechanisms:
Inosine pranobex binds to specific receptors on the cell membrane of lymphocytes or penetrates the cell and has a direct effect on the transmission of signals that increase nuclear-cytoplasmic transport and RNA synthesis in lymphocytes. This leads to the stimulation, maturation and functioning of virus-infected lymphocytes [16].
Inosine pranobex inhibits viral RNA synthesis. This mechanism causes the predominance of mRNA over RNA in the cells of the host organism, which reduces the ability of the virus to control protein synthesis [17].
Inosine pranobex enhances the effect of endogenous γ-IFN, which is one of the components of the stimulating effect of the drug on the cellular immune response. The drug is able to increase the levels of IL-2 and γ-IFN, reduce the level of IL-10, stimulate the maturation and differentiation of T lymphocytes, and also enhance activated lymphoproliferative reactions [15].
In 2021, M. Votava and J. Beran proposed the use of inosine pranobex as a nonspecific immunostimulant in the treatment of infection caused by the SARS-CoV-2 virus [18]. However, descriptions of studies examining the antiviral activity of inosine pranobex against SARS-Cov-2 in vitro
or clinical studies have not yet been found in the available literature.
In 2021, J. Beran et al. published the results of a randomized, placebo-controlled, double-blind, phase IV study demonstrating the efficacy and safety of inosine pranobex in acute respiratory viral infection (ARVI). The study included 463 adult patients with confirmed ARVI, of which 231 received inosine pranobex, 232 received placebo. In the study group, the patients' condition improved faster than in the placebo group; among patients under 50 years of age without obesity, the time for relief of influenza-like symptoms was significantly reduced. Inosine pranobex was found to be well tolerated [19].
Thus, in any epidemic season, there is a problem of choosing antiviral therapy tactics for children with respiratory infections, including recurrent ones. Active forms of HVI, including combined ones, play a role in the etiology of these conditions; therefore, it is necessary to use drugs with a proven wide spectrum of antiviral action.
Purpose of the study
: evaluation of the effectiveness of various treatment regimens using inosine pranobex (Normomed®) in children with monthly infections of the respiratory tract against the background of reactivation of HVI, including combined forms.
Research results
Evaluation of clinical effectiveness
An assessment of the clinical effectiveness of therapy, based on the dynamics of granularity of the posterior pharyngeal wall, hypertrophy of the tonsils, lymphadenopathy and hepatomegaly, is presented in Table 3.
When comparing the severity of granularity of the posterior pharyngeal wall, it is clear that the most pronounced restoration of the mucous membrane of the posterior pharyngeal wall during the therapy was in patients receiving a course of antiviral therapy with inosine pranobex in combination with meglumine credone acetate. In all study groups, changes in the size of the palatine tonsils before and after therapy, as well as in the comparison group, were statistically insignificant. When comparing the degree of lymphadenopathy expressed in points (Mann-Whitney test), it was found that the differences in the groups were statistically insignificant, with the exception of the group with inosine pranobex therapy in combination with recombinant IFN, where statistically significant differences were noted (p = 0.036). The severity of lymphadenopathy was higher before therapy than after therapy (1.5 and 1.0 points, respectively). Thus, the most rapid decrease in the severity of polylymphadenopathy occurred in patients receiving therapy with inosine pranobex in combination with recombinant IFN.
When comparing liver sizes, it was revealed that in the group of patients receiving therapy with a combination of inosine pranobex + recombinant IFN, the indicator before and after therapy had statistically significant differences (p = 0.003 by Student's t-test).
Thus, the clinical effect, assessed using established criteria (see Table 2), was achieved in the majority of patients who received antiviral therapy. The largest proportion of patients with a complete clinical effect, i.e., no respiratory infections for 3 months. from the beginning of observation, was in the group of therapy with inosine pranobex in combination with recombinant IFN - 86%, in the group of monotherapy with inosine pranobex, a complete clinical effect was achieved in 75% of patients, in the group of therapy with inosine pranobex in combination with meglumine acridone acetate - in 63%, and in in the comparison group that did not receive etiotropic therapy - only in 8% of cases. The differences between patients receiving therapy and patients in the comparison group were statistically significant.
Assessment of virological effectiveness
The structure of GVI before and after treatment is presented in Figure 1. In total, before the start of therapy, reactivation caused by HHV-6A/B was recorded in 85.8% of children, caused by EBV - in 35.6%, caused by CMV - in 7.5% caused by HSV-1 - in 16%. Before therapy, monoinfections were diagnosed in more than half of the cases; combined HVIs were diagnosed in 41% of patients; of these, mixed HHV-6A/B + EBV was most often detected - in 23% of cases. After treatment, 63% of patients had a latent infection with HHV-6A/B, the proportion of mixed infections was 8%.
Virological effectiveness was assessed by the proportion of patients with active forms of BBVI in the groups before and after treatment. The proportion of patients with active HHV-6A/B infection after antiviral therapy (65 patients) was 35.4% (23 patients), and in the comparison group (26 patients) this figure was 69.2% (18 patients). When comparing the frequency of HHV-6 reactivation depending on the presence of etiotropic treatment with the drug inosine pranobex (as monotherapy and in combination with other antiviral agents), statistically significant differences were identified (p<0.01). Thus, the use of etiotropic therapy reduces the chances of GVI reactivation by 4.11 times (95% CI 0.092–0.646). There was an average relationship between the compared characteristics (V=0.307).