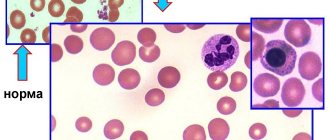
One of the most common diseases in pediatrics is anemia.
In children under 3 years of age, anemia occurs in 40% of cases, during puberty in 30%.
Anemia is a pathological condition characterized by a decrease in hemoglobin content and, in almost all cases, the number of red blood cells in the blood.
Very often, the occurrence of anemia is associated with the intensive growth of the child’s body, the increased process of formation, development and maturation of blood cells in children.
The process of hematopoiesis in a newborn child differs significantly from the process of hematopoiesis in an adult. Only at the 4th - 5th month of life does the bone marrow period of hematopoiesis begin, which becomes decisive for the rest of life. During this period and until the 6th month, there is a physiological decrease in the amount of hemoglobin and red blood cells in the child’s blood. For the normal development of the hematopoietic system, a large amount of iron, vitamins, microelements and protein is required. That is why great importance is given to the child’s nutrition, as well as morbidity during this period. The toxic effects of seemingly the simplest and most easily treatable diseases very often cause the development of anemia in a child.
The most vulnerable are children between the 6th and 12th months of life, which is associated with the depletion of iron reserves accumulated during fetal development.
Long-term anemia in children is accompanied by hypoxia, and profound changes in organs and tissues can develop. Such children lag behind their peers in physical and mental development, they more often suffer from respiratory diseases with the risk of intercurrent diseases (accidentally acquired disease complicating the course of the underlying disease), they are more prone to the development of chronic diseases and various complications.
Causes of anemia.
In order for the process of blood formation in a child to proceed normally, during the period of intrauterine development, his body needs to receive and accumulate a sufficient amount of iron from the mother. At 28-32 weeks of intrauterine development, the most intense transfer of iron from the mother and its accumulation in the child’s body occurs.
Disruption of the normal course of pregnancy during this important period leads to a disruption in the exchange of iron between mother and child and, as a consequence, to its insufficient accumulation in the body.
Maternal anemia and premature birth are the reasons why anemia is detected in all children from birth or from about the 3rd month of life. Multiple pregnancies also lead to the development of anemia.
After birth, anemia can be caused by damage to red blood cells due to hemolytic disease of the newborn and impaired hemoglobin formation.
Most often, anemia is caused by nutritional reasons. In children of the first year of life, anemia develops most often due to monotonous milk feeding, with a poor iron content in breast milk; early transfer of the child to artificial or mixed feeding; using unadapted formulas, cow's or goat's milk for feeding; not timely introduction of complementary foods into the child’s diet.
Premature babies and children born with increased body weight especially need an increased intake of iron into the body.
Therefore, a discrepancy between the intake of iron into the body and its consumption in children of the first year of life can also cause anemia.
Anemia can occur due to blood loss, for example, with frequent nosebleeds. In children with neurodermatitis, food allergies, and exudative diathesis, there is an increased loss of iron from the skin epithelium, so children suffering from such diseases are at risk for developing iron deficiency anemia.
In addition to the direct loss of iron, disturbances in its absorption and metabolism lead to the development of anemia. This can occur, for example: with rickets or lactase deficiency.
The deficiency of B vitamins and microelements is of great importance, which is why a complete and balanced diet is so important.
Any infectious or chronic somatic disease can cause anemia.
Algorithm for diagnosis and treatment of iron deficiency conditions in children
Relevance
Iron deficiency states (IDs) are the most common deficiency conditions in the human population, most often occurring in children and women of reproductive age [1–7]. Thus, it has been established that obvious or hidden iron deficiency occurs in almost half of preschool children and pregnant women [8]. It is noted that the prevalence of VDN is not the same in different countries and depends on social and economic conditions [1]. It should be especially emphasized that the issues of timely diagnosis and adequate treatment of VSD are most acute in pediatric practice. At the same time, the relevance of the problem of iron deficiency in pediatrics is due not only to its widespread prevalence, but also to the significant adverse effect of iron deficiency on the health of children. It has been proven that VDS is the cause of dysfunction of many organs and systems of the body. This is due to the fact that iron is part of many proteins (hemoglobin, myoglobin, cytochromes, iron sulfur proteins, oxidases, hydroxylases, superoxide dismutase, etc.) that ensure systemic and cellular aerobic metabolism and redox homeostasis of the body as a whole. Thus, cytochromes and iron sulfur proteins are necessary for electron transport, and hemoglobin is necessary for oxygen transport. In turn, iron-containing proteins such as oxidases, hydroxylases and superoxide dismutases provide an adequate level of redox reactions in the body [9, 10]. It has been established that insufficient iron content in the body adversely affects metabolic processes, which leads to disruption of the functioning of various organs and systems. At the same time, it has been proven that progressive VHD is accompanied by anemia, impaired growth and development of children, in particular psychomotor development, behavioral changes, decreased intelligence, immune dysfunction and other pathological manifestations [1–9, 11–13].
Reasons for the development of railways
The main causes of iron deficiency syndrome are insufficient intake of iron into the body, poor absorption in the intestines and increased losses. It should be noted that for certain stages of growth and development of the child’s body there are characteristic risk factors for VSD. Thus, in the antenatal period, iron deficiency in the body of the fetus and newborn is caused by: impaired uteroplacental circulation, fetoplacental and fetomaternal bleeding, fetofetal transfusion during multiple pregnancy, intrauterine melena, prematurity, as well as iron deficiency in the mother (long latent). Risk factors for the development of VDS in the intrapartum period are: premature ligation of the umbilical cord, fetoplacental transfusion, hemorrhagic syndrome of various origins (bleeding due to traumatic obstetric procedures or abnormal development of the placenta and umbilical cord vessels) [1, 6]. In the postnatal period, the leading role in the development of VDS in children belongs to the nutritional factor. Thus, in the first year of life, inadequate feeding of a child (use of unadapted milk substitutes for breast milk, late introduction of meat products, insufficient iron content in the diet of children with accelerated growth rates - premature babies, macrosomatics, lymphatics) leads to VDS. In subsequent periods of childhood, nutritional factors can also play a leading role in the development of EDS (eating disorders, veganism, vegetarianism). Particular attention should be paid to the fact that a risk factor for VSD can be an unbalanced diet with insufficient content of meat products in the diet of children who are professionally involved in sports [1, 6]. Impaired absorption of iron from the intestine can be caused by various gastrointestinal diseases (hereditary and acquired malabsorption syndromes, chronic inflammatory bowel diseases, parasitic infestations). Among the main causes of VDS associated with increased losses of iron from the body, it should be noted long-term mild hemorrhage from the gastrointestinal tract with gastrointestinal manifestations of food allergies (in young children - most often to cow's milk proteins), as well as bleeding of various locations and etiologies. At the same time, girls at the period of formation of menstrual function are a particularly high-risk group for the development of VDS [1, 6].
Clinical picture of VDS
The development of the railway network has a staged nature. In this case, tissue iron reserves are first depleted, while in the hematopoietic organs its level remains within standard values. As a result of a decrease in iron content in tissues, the activity of iron-containing enzymes decreases, which is manifested by symptoms of sideropenia. Clinically, dry skin can be noted; brittleness, layering, cross-striations and spoon-shaped nails; glossitis, gingivitis, stomatitis, dysphagia, dyspeptic disorders. It should be emphasized that in the early stages of VDS there are no significant disorders of erythropoiesis, which determines the absence of anemic syndrome during this period. A condition in which a lack of iron in the tissues and organs of the body is not accompanied by anemia is usually called latent iron deficiency
.
In case of further increase in iron deficiency in the body, its content in the hematopoietic organs decreases. This leads to a decrease in hemoglobin synthesis and the development of anemia. At this stage, the symptoms of anemic syndrome (lethargy, weakness, fatigue, decreased physical activity, tachycardia, muffled heart sounds, etc.) are added to the clinical manifestations of sideropenia. The stage of iron deficiency anemia, in which a decrease in iron content in the body is accompanied by impaired erythropoiesis with the development of hypochromic microcytic anemia, is terminologically defined as iron deficiency anemia
(IDA) [1–7].
Diagnostics of the HDS
Taking into account the low specificity of clinical manifestations of sideropenia and anemic syndrome, the diagnostic criteria for latent iron deficiency (LID) and IDA are characteristic laboratory signs. At the same time, it is shown that routine laboratory research methods that are available for wide practice (clinical blood test, determination of iron, ferritin and total iron-binding capacity in the blood serum, followed by calculation of the transferrin saturation coefficient with iron) can be used to verify VDN. It should be especially noted that since ferritin is an inflammatory protein, its determination should be carried out simultaneously with C-reactive protein (CRP) [1–7, 14, 15]. It should be emphasized that for the correct interpretation of a clinical blood test, it is necessary to evaluate all the indicators presented in the hemogram. You cannot limit yourself to only analyzing the level of hemoglobin, leukocytes and ESR. Indicators such as the number of erythrocytes and reticulocytes, erythrocyte indices, “leukocyte formula”, absolute values of neutrophils, lymphocytes, monocytes, eosinophils, platelets, as well as platelet indices must be interpreted. The laboratory criterion for anemia is a decrease in hemoglobin concentration below the age norm. It has been established that the lower limit of normal hemoglobin for children aged 1 month and above. up to 5 years of age the level is 110 g/l, for children 6–12 years old - 115 g/l, for children over 12 years old and adolescents - 120 g/l. It should be noted that already at the first stage of deciphering a clinical blood test, it is possible not only to identify anemia, but also to determine its severity. The severity of anemia is determined by the degree of decrease in hemoglobin (Hb) concentration. Thus, in children older than one month of life, the following approaches are used: a decrease in Hb to 90 g/l is a sign of mild anemia, an Hb level within 70–90 g/l is a marker of moderate anemia, a decrease in Hb below 70 g/l is a criterion for severe anemia. [6]. Identification of laboratory signs of anemia determines the need for mandatory simultaneous assessment of values and other hemogram indicators. If anemia is combined with other changes in the clinical blood test (leukopenia, agranulocytosis, thrombocytopenia), a hematologist must be involved in the supervision of the patient. In those cases when the clinical analysis contains only markers of anemia, the pediatrician can continue to search for the causes of the disease on his own. To do this, the next diagnostic step is to evaluate the erythrocyte indices presented in the blood test (Fig. 1). In this case, first of all, attention is paid to indicators characterizing the degree of saturation of erythrocytes with hemoglobin. These include the color index (CI) and the average hemoglobin content in the erythrocyte - MCH (Mean Corpuscular Hemoglobin). Depending on the degree of saturation of erythrocytes with hemoglobin, anemia is distinguished between normochromic (adequate Hb content in erythrocytes), hypochromic (insufficient Hb content in erythrocytes) and hyperchromic (excessive Hb content in erythrocytes). When hemoglobin decreases below the age norm and signs of erythrocyte hypochromia are detected (CP<0.85, MCH<26 pg), a conclusion is drawn about the presence of hypochromic anemia. Simultaneous analysis of the values of the mean volume of erythrocytes - MCV (Mean Corpuscular Volume) allows you to further characterize the identified anemia as microcytic, normocytic or macrocytic. In cases where there are signs of hypochromic microcytic anemia, it is necessary to make a differential diagnosis between IDA, sideroblastosis and thalassemia. To do this, at the next stage of decoding the hemogram indicators, the level of reticulocytes is assessed (see Fig. 1). If the number of reticulocytes corresponds to standard values (1–2%), this means that anemia is of a normoregenerative nature. In this case, all the characteristics of anemia - hypochromia (CP<0.85, MCH<26 pg) and microcytosis (MCV<80 fL) of red blood cells, normoregeneration (reticulocytes 1-2%) - allow us to conclude that the patient has IDA (see. Fig. 1). It should be especially emphasized that the hypochromic microcytic normoregenerative nature of anemia is typical for IDA of alimentary origin, as well as for those variants of IDA that are caused by impaired absorption of iron in the intestine (malabsorption syndrome, inflammatory bowel diseases, helminthiasis).
In those cases when hypochromic microcytic anemia is accompanied by an increase in the level of reticulocytes (>2%), the hyperregenerative nature of anemia is stated. It should be remembered that these characteristics of anemia can occur both in IDA, which developed as a result of blood loss (posthemorrhagic IDA), and in thalassemia. Let us recall that thalassemia is a group of hereditary diseases caused by a violation of the synthesis of alpha or beta chains of hemoglobin, in which the level of iron in the body not only does not decrease, but in some cases is even increased. It has been established that thalassemia has a clear ethnic connection. Thus, beta thalassemia is most often found in people from the Mediterranean, Middle East and India, and alpha thalassemia is most often found in people from Africa and Southeast Asia. Taking this into account, when identifying hypochromic microcytic anemia in a child with the specified ethnicity, in addition to searching for typical clinical (mild icterus, hepatosplenomegaly) and laboratory (targeted erythrocytes) manifestations of thalassemia, it is necessary to detail the family history, paying special attention to the health status of immediate relatives ( chronic anemia, hepatosplenomegaly, cholelithiasis from a young age, etc.) [17–20]. Thus, when identifying hypochromic microcytic hyperregenerative anemia, it is necessary to make a differential diagnosis between IDA of posthemorrhagic origin and thalassemia (see Fig. 1). Since with thalassemia, unlike IDA, the iron content in the body does not decrease, to clarify the genesis of the disease in this case it is necessary to examine the child’s ferrostatus. To do this, the level of iron (IF), ferritin (FS), total iron-binding capacity (TIBC) is determined in the blood serum, and the coefficient of transferrin saturation with iron (TISC) is calculated. Simultaneously with PS, it is advisable to study CRP, since ferritin is an inflammatory protein and its value may have a false positive level during the inflammatory process in the body. If, when examining a child with hypochromic microcytic hyperregenerative anemia, normal or increased levels of vitreous acid, PS (with normal values of CRP) and CNTG are detected, then it is necessary first of all to assume the presence of thalassemia. For this, the patient is referred for a consultation to a hematologist, who conducts a study of the osmotic resistance of erythrocytes, hemoglobin electrophoresis, and, if necessary, recommends a genetic examination. If hypochromic microcytic hyperregenerative anemia is accompanied by a decrease in SF (<14 µmol/l), FS (<12 µg/l), LTFA (<17%) and a simultaneous increase in CVS (>63 µmol/l), then IDA of post-hemorrhagic origin occurs ( see Fig. 1).
Diagnostics of latent VDNs
Laboratory criteria for a decrease in iron content in the body (SF<14 µmol/l, FS<12 µg/l, CNFT<17%, TIAC>63 µmol/l) are used not only for diagnosing IDA, but also for verifying LVAD (Table 1 ). It should be recalled that LVDS is the initial stage of deficiency, in which tissue reserves of iron in the body are depleted, but there are still no deep disturbances in erythropoiesis, which explains the absence of anemic syndrome. As a result, laboratory changes in LVDS are characterized only by features of ferrostatus typical of iron deficiency and are not accompanied by signs of anemia. Since the clinical signs of LVDS are nonspecific, and anemic syndrome in the early stages of iron deficiency is absent in the body, in the vast majority of cases LVDS is not diagnosed. In this regard, the true prevalence of CVD in the pediatric population (including in Russia) remains unspecified. At the same time, the results of pilot studies indicate that in target groups and/or in certain regions of our country, the incidence of CVD can reach 40% [5–7, 20–24]. In this regard, the relevance of screening for the timely detection of LVAD is beyond doubt. However, it is clear that in routine pediatric practice, screening for VDN, based on determining ferrostatus in venous blood, cannot be implemented in the vast majority of cases. In this regard, alternative methods for the early diagnosis of LVAD, based on the study of new clinical blood test indicators, are currently being actively studied [6, 16, 23, 24].
The emergence of the latest generation of hematological analyzers in practical healthcare allows, when studying a clinical blood test, to additionally determine the degree of saturation of reticulocytes with hemoglobin (Ret-Hb indicator) and the difference between the hemoglobin content in mature erythrocytes and in reticulocytes (Delta-Hb indicator) [25]. It has been established that with the help of a correct assessment of these indicators, it is possible to identify initial disturbances in hemoglobin synthesis in LVDS. Thus, if children at risk for the development of iron deficiency do not have anemia (Hb concentration is within normal limits), and red blood cells are characterized by normochromia (MCH = 26–32 pg) and normocytosis (MCV = 80–95 fL), then special attention should be paid to the assessment indicators such as Ret-Hb and Delta-Hb. At the same time, the detection of a decrease in the average hemoglobin content in reticulocytes (Ret-Hb <26 pg) and an increase in differences in the hemoglobin saturation of erythrocytes and reticulocytes (Delta-Hb >2 pg) allows us to conclude that the child has significant CVD with initial signs erythropoiesis disorders. This conclusion is based on the fact that reticulocytes carry the “latest” information about the state of erythropoiesis, since they are detected in the bloodstream only during the first day after leaving the bone marrow, and the average lifespan of erythrocytes is 120 days. Thus, the detection of low values of saturation of reticulocytes with hemoglobin (Ret-Hb <26 pg) and an increase in the level of Delta-Hb, with the simultaneous absence of anemia, hypochromia and microcytosis of erythrocytes, is a marker of LVDS (Fig. 2). In diagnostically difficult cases, laboratory indicators such as zinc protoporphyrin and soluble transferrin receptors, characterized by high sensitivity and specificity, can be used to verify VDN, but their determination is not widely available in practice [26, 27].
VDN therapy
The basis of therapy for VDD, both latent and manifest, is the rational use of iron supplements. In the vast majority of cases, oral forms of iron supplements are used to treat VDD. It should be noted that in the modern medicinal arsenal of a pediatrician there are iron preparations of different composition (salt, hydroxide polymaltose complexes) and release form (tablets, capsules, syrups, drops). In this case, the calculation of individual doses of drugs, regardless of the form of release, should be carried out taking into account the content of elemental iron in the drug. Thus, if iron preparations are used in the form of a polymaltose hydroxide complex, then the daily dose of elemental iron should be 5 mg/kg per day. In cases where iron salt preparations are used, the following daily doses of elemental iron are recommended: children under 3 years of age - 3 mg/kg per day; children over 3 years old - 45–60 mg/day; adolescents - up to 120 mg/day. Tardiferon
can be successfully used for the treatment of LVDS and IDA [28]. Tardiferon is a long-acting drug in which iron is presented in the form of ferrous sulfate [28]. It should be noted that the results of studies of the pharmacokinetic characteristics of the updated formula of the drug Tardiferon [29, 30] indicate a prolonged release of iron in the gastrointestinal tract. It has been shown that the prolonged release of iron from the drug contributes to its optimal absorption and good tolerability [29, 30]. Confirmation of the good tolerability of the drug Tardiferon, as well as its high clinical and economic effectiveness, was also obtained in a series of domestic studies [31, 32]. 1 tablet of the drug Tardiferon contains 80 mg of elemental iron, which allows us to recommend the following dosage regimen: children 6–10 years old - 1 tablet per day; children over 10 years of age and adolescents - 1–2 tablets per day [28]. The drug Tardiferon is taken before meals (without chewing), with water, or during meals. It should be remembered that Tardiferon is not recommended for use simultaneously with antacids and tetracyclines. In addition, it is necessary to take into account that the absorption of iron in the intestines decreases with the simultaneous use of products containing polyphenols (beans, nuts, tea, coffee), phytates (grains, legumes, vegetables, nuts) and a large amount of dietary fiber. With a correctly established diagnosis of IDA, an adequate choice of iron supplements and a correctly selected dose, a clinical blood test shows an increase in reticulocytes on the 10-14th day of therapy, and by the end of the 3-4th week of treatment - an increase in hemoglobin by 10 g/l. Normalization of hemoglobin levels is achieved after 4–8 weeks. from the start of therapy. In cases where, against the background of adequately administered therapy within the prescribed time frame, there are no expected positive changes in the clinical blood test, one should doubt the correctness of the diagnosis. Considering that hypochromia of erythrocytes is characteristic not only of IDA, but also of sideroblastosis and thalassemia, it is necessary to discontinue iron supplements and return to the diagnostic search for the causes of anemia. We consider it necessary to pay attention to another very important aspect - strict adherence to the recommended duration of use of iron supplements in the treatment of VSD. Unfortunately, in practice these recommendations are not always strictly followed. Normalization of hemoglobin levels, which is usually observed within 1–2 months. from the start of treatment should not be a reason to discontinue iron supplements. This is due to the fact that relief of anemia only indicates normalization of erythropoiesis, while iron deficiency in tissue depots still persists. In other words, the relief of IDA is the removal of only the tip of the iceberg, while its main part (IDA) is hidden under water. In this regard, it is necessary to strictly adhere to the following rule: the duration of treatment with iron supplements is determined by the severity of VDD and the severity of anemia. Thus, for a mild degree of IDA, the course of treatment with iron supplements is 3 months, for a moderate degree - 4.5 months, for a severe degree - up to 6 months. [6].
Conclusion
In conclusion, it is advisable to note once again that early diagnosis of VSD, their timely and adequate correction with the help of iron supplements will significantly reduce the risk of disruption of the functioning of various organs and systems of the growing body, which will not only have a positive impact on the child’s health, but will also improve the quality of his life generally.
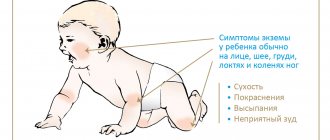
Symptoms of anemia in children
Visible changes in anemia in children are observed in the skin: it becomes pale, dry, flaky; nails may become deformed and brittle; hair loses its healthy shine.
In severe forms of anemia and its long-term course, children develop cracks on the palms of the hands and soles of the feet, and in the corners of the mouth. Children with anemia are weakened, often suffer from acute respiratory viral infections, bronchopulmonary diseases, and are susceptible to acute intestinal infections.
The lack of oxygen that occurs with anemia also affects the nervous system. The child becomes whiny, lethargic, gets tired quickly, may experience dizziness, and muscle tone decreases.
The digestive system also reacts. After feeding, children experience frequent regurgitation and vomiting, flatulence, possible diarrhea or constipation, enlarged spleen and liver, and decreased appetite.
Prevention of anemia
Prevention begins with good nutrition for the pregnant woman, allocating sufficient time for walking and sleeping. Children of the first year of life need to add complementary foods to breast milk after 6 months of age; for older children, a varied, balanced diet should be organized. It is important that during the warm season the baby spends as much time outdoors as possible and takes sunbathing. The team of doctors at the SM-Doctor clinic will help your baby cope with anemia and develop individual preventive measures. Contact professionals to create ideal conditions for the growth and development of your child!
Diagnosis of anemia.
The basis for diagnosing anemia is laboratory tests, namely a clinical blood test.
The normal blood hemoglobin level for children under 6 years of age is 125–135 g/l;
Anemia in a child is indicated if this indicator drops below 110 g/l (in children under 5 years old) and below 120 g/l (in children over 5 years old).
A general blood test for anemia in children reveals a decrease in hemoglobin (less than 120-110 g/l), a decrease in red blood cells (<3.8x1012/l).
A biochemical blood test includes determining such indicators as:
- serum iron;
- transferrin saturation with iron;
concentrations:
- ferritin;
- bilirubin;
- vitamins
Treatment of anemia
Methods for correcting anemia depend on age.
When breastfeeding, it is necessary to introduce vegetable, fruit and meat complementary foods on time, and spend a lot of time in the fresh air, including in the sun. The room must be well ventilated, kept clean, and sanitary rules for child care must be observed. For older children, it is enough to streamline their diet and daily routine to improve their blood tests. The diet includes beef liver, legumes, seafood, fresh vegetables and fruits, and herbs. If necessary, the timing of vaccination is postponed, massage and ultraviolet irradiation are used.
Medicines are prescribed by a doctor. Iron supplements and vitamins are indicated. The minimum duration of drug treatment is 1 month.
Causes of B12 deficiency anemia
A lack of vitamin B12 may be due to the following reasons or a combination of them:
- impaired absorption of the vitamin in the intestine: gastritis associated with Helicobacter pylori infection, Crohn's disease, previous intestinal surgeries, polyposis of the gastric mucosa, cancer, intestinal fistulas, etc.;
- congenital metabolic disorders;
- chronic intoxication with nitric oxide, used as sedation in anesthesiology.
- minor absorption disorders, for example, a decrease in the availability of B12 in food with chronic pancreatitis, moderate atrophic gastritis, taking metformin, medications to reduce the production of hydrochloric acid, etc.;
- poor diet, vegetarianism;
- chronic alcoholism.
One form of B12 deficiency anemia is Addison-Biermer disease. With it, autoantibodies appear against the cells of the gastric mucosa.
It is worth noting that B12 and folate deficiency anemias are closely related. Folic acid (vitamin B9) also takes part in the formation of red blood cells. Even a full supply of these components in food does not guarantee the absence of anemia in cases where there is an increased consumption or there is a violation of utilization in the bone marrow. This may be due to parasitosis, intestinal dysbiosis, pregnancy, liver and blood diseases.
Pathogenesis (what happens?) During iron deficiency anemia in a child:
Iron leaves the body through urine, feces, sweat, as well as through nails, hair and skin. Losses do not depend on gender. In children, about 0.1–0.3 mg is released into the external environment per 24 hours. In adolescents, the loss is a maximum of 0.5–1.0 mg. The child needs to receive 0.5–1.2 mg of iron per day. There is an increased need for infants and preschoolers. In order for a child to develop normally, the diet should contain 1.5 mg of iron per day in newborns, and at least 10 mg in children from 1 to 3 years old.
Iron deficiency provokes a tendency to infectious diseases of the baby's gastrointestinal tract and respiratory system. Without iron, brain structures cease to function normally. With a lack of iron, disruptions occur in the neuropsychic development of the baby. In children who were diagnosed with iron deficiency anemia in infancy, at the age of 3-4 years, disturbances in the transmission of nerve impulses from the centers of the brain to the organs of hearing and vision are found due to impaired myelination and, as a consequence, impaired nerve conduction.
Diagnostics
Diagnosis of iron deficiency anemia in infants and older children in the multidisciplinary CELT clinic is carried out with the participation of various specialists:
- pediatrician;
- hematologist;
- pediatric gastroenterologist.
The child is examined, a medical history is taken, and a laboratory examination is performed, which makes it possible to establish a diagnosis of anemia and determine its degree. For the purpose of additional examination, it is possible to use an ultrasound diagnostic method (abdominal organs, pelvic organs).
Diagnostic features
Treatment of B12-deficiency anemia is prescribed after a comprehensive examination. The doctor will order the following laboratory hematological and other tests:
- general blood analysis;
- biochemical and immunological studies: determination of the level of B12 in plasma, assessment of the level of homocysteine in serum or blood plasma, determination of antibodies to gastric parietal cells, etc.;
- bone marrow aspiration or trephine biopsy.
Gastroscopy may also be required to detect atrophic gastritis.
The diagnosis is made by a doctor based on complaints, manifestations of the disease, and laboratory test parameters. It is important to find out the cause of the syndrome, especially to detect Addison-Biermer disease.
In addition, a specialist needs to distinguish this type of anemia from other diseases accompanied by disturbances in hematopoietic processes. Thus, if liver cirrhosis is suspected, disorders associated with long-term use of certain drugs (sulfonamides, anticonvulsants), sideroblastic anemia will require differential diagnosis.