Buy Buserelin-long lyophilisate for the preparation of suspension intramuscularly 3.75 mg in pharmacies
Buserelin-long FS Buy Buserelin-long FS in pharmacies DOSAGE FORMS lyophilisate for preparing a suspension for intramuscular injection. entered prolong. active 3.75 mg
MANUFACTURERS F-Sintez (Russia)
GROUP Antiandrogens
COMPOSITION The active substance is buserelin.
INTERNATIONAL NON-PROPENTED NAME Buserelin
SYNONYMS Buserelin, Buserelin FSintez, Buserelin-depot, Buserelin acetate solution
PHARMACOLOGICAL ACTION Antigonadotropic, antiandrogenic, antiestrogenic. Competitively binds to the receptors of the cells of the anterior pituitary gland (it is a synthetic analogue of gonadotropin-releasing hormone) and completely blocks its gonadotropic function within 12-14 days. Inhibits the release of luteinizing hormone (LH) and follicle-stimulating hormone (FG). There is a suppression of the synthesis of sex hormones in the ovaries and a decrease in the concentration of estradiol in the blood to postmenopausal values; the testosterone concentration in the testicles decreases to the post-castration level. It is well absorbed from the nasal mucosa and after subcutaneous administration.
INDICATIONS FOR USE Hormone-dependent prostate cancer stages III and IV (if it is necessary to inhibit testosterone production in the testes); breast cancer in women with a preserved menstrual cycle and the presence of estradiol/progesterone receptors; hormone-dependent pathology of the reproductive system, caused by absolute or relative hyperestrogenism (endometriosis, uterine fibroids, endometrial hyperplastic processes); for induction of ovulation in the treatment of infertility (in combination with gonadotropins) in in vitro fertilization (IVF) programs.
CONTRAINDICATIONS Hypersensitivity, pregnancy, lactation.
SIDE EFFECTS: Headache (with intranasal administration), mood lability, sleep disturbance, depression, symptoms of eye irritation (when wearing contact lenses); change in appetite, nausea, vomiting; decreased libido, impotence, vaginal dryness, ovarian cysts, pain in the lower abdomen, menstrual bleeding; allergic reactions (urticaria, skin hyperemia, angioedema); hot flashes, irritation of the nasal mucosa and nosebleeds (with intranasal administration), increased sweating (with intranasal administration), acne, dry skin and mucous membranes, bone demineralization, gynecomastia, thrombosis, swelling of the feet and ankle joints; symptoms associated with an increase in testosterone levels in the blood at the beginning of therapy (bone pain, numbness or tingling sensation in the hands or feet, difficulty urinating, weakness in the legs).
INTERACTION With the simultaneous use of hormonal contraceptives or other sex hormone preparations, the development of ovarian hyperstimulation syndrome is possible. Reduces the effect of hypoglycemic drugs.
OVERDOSE No information available.
SPECIAL INSTRUCTIONS Before starting treatment, it is necessary to exclude pregnancy; during the first 2 months of therapy, adequate contraception is necessary. Prescribe with caution to patients with depression. In the initial stage of treatment, the development of an ovarian cyst is possible. Use with caution while working for vehicle drivers and people whose profession involves increased concentration.
STORAGE CONDITIONS List B. In a place protected from light, at a temperature of 8-20°C.
Buserelin depot in the treatment of prostate cancer
G.P. Kolesnikov
GAUZ "Moscow City Oncology Hospital No. 62" of the Moscow Department of Health Russia, 143423, Moscow region, Krasnogorsky district, Stepanovskoye p/o, pos. Istra, 27
Contacts:
Gennady Petrovich Kolesnikov, e-mail: .
Print version
The high incidence of prostate cancer (PCa) and the very widespread use of hormonal therapy for its treatment as an independent method for common forms (T3-T4, N1, M1), relapses and progression of the disease after local treatment methods (radical prostatectomy, radiation therapy), as well as in combined treatment as neoadjuvant and adjuvant therapy, they determine the importance of a sufficient set of hormonal drugs in the urologist’s arsenal. Among the luteinizing hormone releasing hormone (LHRH) agonists, buserelin has been known for a long time, which is produced in Russia and whose clinical effectiveness is not inferior to imported analogues. The results of a study of the effectiveness and safety of treatment with buserelin depot in 20 patients with morphologically verified prostate cancer aged from 53 to 87 years are presented; the duration of the study was 3 months. During therapy with buserelin depot in patients with prostate cancer, a consistent decrease in the average values of prostate-specific antigen (PSA) was revealed from 81.2 to 27.8 ng/ml after 2 months and to 23.0 ng/ml after 3 months. During the therapy, a decrease in testosterone levels to post-castration values was achieved from the initial 168 to 21 ng/dl after 1 month and to 19.8 ng/dl after 3 months of treatment. All patients underwent treatment with buserelin depot without clinically significant adverse reactions. The most common complaints were increased sweating and hot flashes, characteristic of all drugs of the LHRH agonist group, no more pronounced than in treatment with other drugs of this group.
Conclusions.
Buserelin-depot at a dose of 3.75 mg is a highly effective domestic drug for the treatment of hormone-dependent prostate cancer. The use of buserelin depot leads to a decrease in PSA levels and ensures a stable decrease in serum testosterone levels to post-castration levels in the absence of serious side effects. The results obtained do not differ from similar indicators when using imported analogues of LHRH.
Keywords:
prostate cancer, hormonal therapy, luteinizing hormone-releasing hormone agonists, buserelin, prostate-specific antigen, testosterone, effectiveness and safety of therapy
DOI: 10.17 650/1726-9776-2015-11-3-&-&
GP Kolesnikov
Moscow City Cancer Hospital Sixty-Two, Moscow Healthcare Department; Stepanovskoe, 27, Istra Settlement, Krasnorgorsky District, Moscow Region 143423, Russia
The high incidence of prostate cancer (PC) and considerably the wide use of hormone therapy for its treatment as an individual modality for its advanced forms (T3-T4, N1, M1), recurrences, and progression after local treatments (radical prostatectomy, radiation therapy) and for combined treatment as neoadjuvant and adjuvant therapies determine the importance of a sufficient range of hormonal drugs at an urologic oncologist's disposal. Among the luteinizing hormone-releasing hormone (LHRH) agonists, there is rather long-known buserelin made in Russia, the clinical efficacy of which is highly competitive with foreign analogs.
The paper presents the results of a 3-month trial of the effectiveness and safety of buserelin depot treatment in 20 patients aged 53 to 87 years with morphologically verified PC. The patients with PC showed a gradual decrease in the mean values of prostate-specific antigen (PCA) from 81.2 to 27.8 and 23.0 ng/ml at 2 and 3 months of buserelin depot therapy, respectively. The performed therapy could achieve reductions in testosterone levels from 168 ng/dl at baseline to 21 and 19.8 ng/dl postcastration at 1 and 3 months of the therapy.
All the patients tolerated buserelin depot therapy without having clinically significant adverse reactions. The most common complaints were hyperhidrosis and hot flushes, which are typical of all LHRH agonists and which are not marked in the treatment with other drugs of this group.
Conclusion.
Buserelin depot 3.75 mg is a highly effective Russian drug to treat hormone-dependent PC. Its administration causes a reduction in PSA levels and ensures a steady decline in serum testosterone concentrations to the postcastration level without causing serious side effects. The findings do not differ from those when foreign LHRH analogues are used.
Key words:
prostate cancer, hormone therapy, luteinizing hormone-releasing hormone agonists, buserelin, prostate-specific antigen, testosterone, efficiency and safety of therapy
Introduction
Prostate cancer (PCa) is a common malignancy in men. In Europe, it is the most common solid cancer, with an incidence of 214 cases per 1000 men. Every year, more than 800 thousand new cases of prostate cancer are detected worldwide [1, 2].
The incidence of cancer of this localization in Russia has been rapidly increasing over the past 2 decades. In 2011, 28,552 new cases of prostate cancer were registered, in 2012 – more than 29,000. In the structure of cancer incidence, prostate cancer took 2nd place. In terms of growth rate, it is significantly ahead of malignant tumors of other localizations. The mortality rate also remains high [3, 4].
In Russia, there has been a trend towards an increase in the identification of patients with a localized form of prostate cancer. However, despite this, half of the patients are initially diagnosed with locally advanced and metastatic forms of prostate cancer [5]. Hormone therapy for prostate cancer, aimed at blocking testosterone synthesis, is widely used to treat primary advanced forms of prostate cancer, as well as relapses after local treatment methods (radical prostatectomy and radiation therapy) [4, 6, 7].
The main types of hormone therapy (androgen deprivation) today are surgical castration (bilateral orchiectomy) and medical castration - luteinizing hormone releasing hormone agonists (LHRH) and antagonists (firmagon) [4, 7]. Surgical castration has not been canceled or prohibited, but medical castration is more often used in practice due to the choice of the patient and the availability of drugs for its implementation, as well as the possibility of having a reversible effect upon cancellation. Currently, there are 4 main classes of substances that can be classified as LHRH agonists: goserelin, triptorelin, buserelin and leuprolide [7].
All drugs of the LHRH agonist group have approximately the same effectiveness in the treatment of prostate cancer, similar adverse events associated with low testosterone levels (hot flashes, sweating, decreased libido and potency, etc.) [4, 7]. But to date, only buserelin is produced in Russia, although it was synthesized as one of the first agonists [8]. Studies of Russian-made buserelin presented in the literature have shown its effectiveness and tolerability to be the same as that of imported analogues [4, 8-10].
The purpose of the study was to evaluate the effectiveness and safety of treatment of patients with prostate cancer with the drug buserelin-depot (ZAO Pharm-Sintez, Russia).
Materials and methods
From February to May 2014, at the clinic of the Moscow City Oncology Hospital No. 62, 20 patients with morphologically diagnosed prostate cancer were treated with buserelin depot. The age of the patients ranged from 53 to 87 years, the average age was 69.8 years.
Buserelin depot at a dose of 3.75 mg was used for 3 cycles with intramuscular administration once every 28 days.
In all patients, the diagnosis of prostate adenocarcinoma with varying degrees of differentiation according to the Gleason score (Gleason index) was previously morphologically confirmed. During histological verification of the diagnosis, moderate and poorly differentiated forms of PCa predominated (Gleason index 6–10).
All patients at the start of treatment had a prostate-specific antigen (PSA) level above normal. Before treatment with buserelin depot, the average PSA value was 81.2 (4.2–679) ng/ml. Half of the patients (n = 10) had distant metastases, represented mainly by bone deposits confirmed by bone scintigraphy, X-ray or magnetic resonance imaging (MRI). The number of bone metastases ranged from 2 to total damage to the bone skeleton. The distribution of patients by disease stage was as follows: T2N1M0 – 1, T3N0M0 – 9, T3N0M1 – 7, T3N1M1 – 2, T4N1M1 – 1 patient.
Based on the extent of the tumor, hormone therapy was prescribed to all. In some patients with bone metastases, in addition to hormonal treatment, other types of specialized treatment were used: palliative external beam radiation therapy for bone metastases, treatment with zoledronic acid, etc.
11 patients had pain, 9 did not require pain relief.
The effectiveness of drug treatment was assessed once a month. The examination included determination of PSA levels, serum testosterone, assessment of general condition according to the Karnofsky scale and pain syndrome according to the World Health Organization scale (clinical response).
Results and discussion
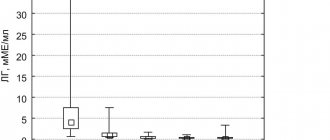
When analyzing the results obtained, it was found that the majority of patients receiving buserelin depot had positive PSA dynamics, characterized by a sharp decrease in the first 2 months of treatment, after which a gradual regression and stabilization of indicators occurred.
During therapy with buserelin depot in patients with prostate cancer, a consistent decrease in mean PSA values was revealed from 81.2 to 27.8 ng/ml after 2 months, to 23.0 ng/ml after 3 months.
During the therapy, a decrease in testosterone levels to post-castration values was achieved from the initial average of 168 to 21 ng/dl after 1 month and 19.8 (3–29) ng/dl after 3 months of treatment.
A decrease in the level of pain was clinically noted in 4 (36.3%) patients out of 11 who had pain, which allowed 2 of them to stop using narcotic analgesics.
Since the group of elderly patients under observation (average age about 70 years), in half of the cases with metastatic prostate cancer, the severity of their condition, in addition to the underlying cancer, was due to concomitant age-related pathology. The choice of a drug for long-term therapy dictates the need not only for its effectiveness, but also for a minimum number of side effects and good tolerability.
All patients underwent treatment with buserelin depot without clinically significant adverse reactions. The most common complaints were increased sweating and hot flashes, characteristic of all drugs of the LHRH agonist group, no more pronounced than with treatment with other drugs of this group. No allergic reactions were registered. There were no cases of urinary retention, muscle weakness in the lower extremities, edema or lymphostasis. No local reaction to the administration of the drug was observed. Not a single patient refused treatment due to poor tolerance.
It should be noted that the majority of patients, after completing the 3-month study, the results of which are presented here, remain on hormone therapy with buserelin depot in a constant or intermittent mode, with good tolerability and response observed according to clinical data and PSA levels with varying durations.
conclusions
- Buserelin-depot is a highly effective domestic drug for the treatment of hormone-dependent prostate cancer.
- The use of buserelin depot leads to a decrease in PSA levels and ensures a stable decrease in serum testosterone levels to post-castration levels in the absence of serious side effects. The results obtained do not differ from similar indicators when using imported analogues of LHRH.
- Buserelin depot can be recommended for use as independent therapy or in combination with other treatment methods in patients with prostate cancer.
Literature
- Anderson J., Abrahamsson PA., Crawford D. et al. Management of advanced prostate cancer: can we improve on androgen deprivation therapy. BJU Int 2008;101:1497-501.
- Heidenreich A., Pfister D., Ohlamann CH et al. Androgen deprivation for advanced prostate cancer. Urologe 2008;47:270-83.
- Malignant neoplasms in Russia in 2012 (morbidity and mortality). Ed. HELL. Kaprina, V.V. Starinsky, G.A. Petrova. M.: FSBI “MNIOI im. P.A. Herzen" Ministry of Health of Russia, 2014. .
- Mishugin S.V., Mordovin A.A., Gritskevich A.A., Rusakov I.G. Medical castration in the treatment of common forms of prostate cancer. The role of buserelin. Medical Council 2014;8:3-6.
- Kaprin A.D., Starinsky V.V., Petrova G.V. The state of cancer care for the population of Russia in 2012. M., 2013.
- Babaev R., Matveev V.B., Volkova M.I. Predictive factors for survival in patients with advanced prostate cancer receiving hormone therapy. Oncourology 2011;2:78-83.
- Nyushko K.M., Alekseev B.Ya., Kalpinsky A.S., Kaprin A.D. Antagonists of luteinizing hormone-releasing hormone in patients with prostate cancer. Standard approach and results of innovative research. Oncourology 2014;4:70-4. [Nyushko KM, Alexeev BY, Kalpinsky AS, Kaprin AD Luteinizing hormone-releasing hormone antagonists in patients with prostate cancer. Standard approach and results of innovational studies. Onkourologiya = Oncourology 2014;4:70-4. (In Russ.)].
- Rapoport L.M., Demidko Yu.L. Use of buserelin depot, a gonadotropin-releasing hormone agonist, in the treatment of prostate cancer. Andrology and Genital Surgery 2014;3:2-7.
- Sivkov A.V., Matveev V.B., Bukharkin B.V. and others. Results of long-term use of the gonadotropin-releasing hormone agonist buserelin-depot in patients with prostate cancer. Consilium Medicum 2005;7(7):591-5.
- Kopyltsov E.I., Leonov O.V., Krivonogov I.I. and others. Domestic deposited drug in the treatment of patients with prostate cancer. Oncourology 2005. Abstracts of the 6th All-Russian. scientific-practical conference “Current issues in the treatment of urological cancer diseases”. pp. 94-5.
Contacts
: Kolesnikov Gennady Petrovich, MD, professor, head of the department of oncourology of the polyclinic of the Moscow City Oncology Hospital No. 62. Tel., email: .