Pharmacological properties of the drug Bivalos
Strontium ranelate (5-[bis(carboxymethyl) amino] 2-carboxy-4-cyano-3-thiophenacetic acid, distrontium salt) has a dual mechanism of action and is indicated for the treatment of postmenopausal osteoporosis to reduce the risk of vertebral and femoral fractures. In vitro studies have found that strontium ranelate increases bone formation in bone tissue culture, as well as the proliferation of osteoblast precursors and collagen synthesis in bone cell culture; reduces bone resorption by reducing the differentiation of osteoclasts and reducing their resorptive activity. The dual mechanism of action leads to a rebalancing of metabolic processes in bone tissue in favor of osteogenesis. In experimental studies, strontium ranelate increased trabecular bone mass, trabecular number, and trabecular thickness. This led to increased bone strength. Clinical and experimental studies have demonstrated that strontium in bone tissue is mainly adsorbed on the surface of apatite crystals and only in small amounts replaces calcium in apatite crystals in newly formed bone tissue. Strontium ranelate does not alter the characteristics of bone crystals. In a study of iliac crest bone biopsies obtained up to 60 months after initiation of strontium ranelate therapy at a dose of 2 g/day, no adverse effects on bone quality or mineralization were noted. The average bone mineral density (BMD) during treatment with strontium ranelate increases compared to the baseline level by approximately 4% per year in the lumbar spine and by 2% per year in the femoral neck, reaching after 3 years (according to various studies) increases on average by 13–15% and 5–6%, respectively. When using strontium ranelate compared with placebo, starting from the 3rd month of treatment and throughout 3 years of therapy, an increase in the level of biochemical markers of bone formation (bone-specific ALP and C-terminal propeptide of procollagen type I) and a decrease in the level of biochemical markers of resorption are noted bone (serum C-telopeptide and cross-linked N-telopeptide in urine). In addition, a slight decrease in the concentration of calcium and parathyroid hormone (PTH) in the blood serum, an increase in the concentration of phosphorus in the blood and the total activity of alkaline phosphatase without any clinical manifestations were noted. Strontium ranelate consists of two stable strontium atoms and a ranelic acid molecule. The organic part of the compound provides better characteristics of the molecular weight, pharmacokinetics and tolerability of the drug. Due to the high polarity of the compound, a low degree of absorption, tissue distribution and binding of ranelic acid to blood plasma proteins is noted. Ranelic acid does not accumulate in the body; there is also no data indicating its metabolic transformations in the body of animals and humans. After absorption, ranelic acid is quickly excreted unchanged in the urine. The absolute bioavailability of strontium after oral administration of 2 g of strontium ranelate is approximately 25% (range 19–27%). The maximum concentration in blood plasma is achieved 3–5 hours after a single dose of 2 g. Equilibrium concentration is achieved 2 weeks after the start of treatment. Simultaneous administration of strontium ranelate with food or calcium salts reduces the bioavailability of strontium by approximately 60–70% compared to taking the drug 3 hours after a meal. Due to the relatively slow absorption of strontium, it is necessary to avoid food and calcium supplements both before and after taking strontium ranelate. Oral vitamin D supplements do not affect strontium concentrations. The volume of distribution of strontium is about 1 l/kg. The binding of strontium to human plasma proteins is low (25%), strontium has a high affinity for bone tissue. When determining the concentration of strontium in biopsy samples of the iliac crest of patients who were treated with strontium ranelate at a dose of 2 g/day up to 60 months inclusive, it was found that the value of strontium concentration in bone tissue can reach a plateau 3 years after the start of treatment. There are no data on the kinetics of strontium excretion from bone tissue after the end of therapy. As a divalent cation, strontium is not metabolized in the body. Strontium ranelate does not inhibit enzymes of the cytochrome P450 system. Strontium excretion depends on dose and time. The effective half-life of strontium is about 60 hours. Strontium is eliminated from the body in urine and feces. Its clearance from blood plasma is about 12 ml/min (coefficient of variation is 22%), and renal clearance is about 7 ml/min (coefficient of variation is 28%). Population pharmacokinetic data indicate that there is no relationship between age and the rate of strontium excretion in the target group of patients. Data on pharmacokinetics in patients with impaired liver function are not available, however, given the pharmacokinetic properties of strontium, no characteristic effect can be expected. The research program for the drug's effectiveness in preventing bone fractures consisted of two placebo-controlled studies: the SOTI study and the TROPOS study. The SOTI study included 1649 postmenopausal women with an established diagnosis of osteoporosis (with low BMD of the lumbar spine and a history of fractures in more than half of the women); the average age of the study participants was 70 years. The TROPOS study included 5091 postmenopausal women with an established diagnosis of osteoporosis (low BMD at the femoral neck and a history of fractures in more than half of the women); the average age of the study participants was 77 years. Both studies included 1556 patients aged over 80 years at the time of enrollment (accounting for 23.1% of the entire study population). In addition to strontium ranelate 2 g/day or placebo, patients took calcium and vitamin D supplements throughout the study period. Bivalos reduced the relative risk of developing new vertebral fractures over 3 years by 41% (SOTI study, Table 1 ). The effect was statistically significant starting from the 1st year of the study. Similar benefits were demonstrated in women with multiple fractures before the start of the study. The relative risk of symptomatic vertebral fractures (defined as fractures accompanied by back pain and/or a decrease in height of at least 1 cm) decreased by 38%. The use of Bivalos compared to placebo was also accompanied by a decrease in the number of cases of height loss of at least 1 cm. The results of studying the quality of life using an assessment using a special QUALIOST scale, as well as a scoring scale of general well-being based on the general scale of the SF-36 questionnaire, demonstrated the advantages of the drug compared with placebo. The effectiveness of the drug in reducing the risk of developing new cases of vertebral fractures was confirmed in the TROPOS study, including in patients with osteoporosis who did not have fractures before treatment. Table 1 Number of patients with vertebral fractures and a decrease in the relative risk of their development
SOTI Study | Placebo | Bivalos | Relative risk reduction compared with placebo (95% confidence interval), p value |
n=723 | n=719 | ||
| New vertebral fracture during 3 years of treatment | 32,8% | 20,9% | 41% (27–52), p≤0.001 |
| New vertebral fracture during the 1st year of treatment | 11,8% | 6,1% | 49% (26–64), p≤0.001 |
| New vertebral fracture with clinical symptoms during 3 years of treatment | 17,4% | 11,3% | 38% (17–53), p≤0.001 |
| TROPOS Study | n=1823 | n=1817 | |
| New vertebral fracture during 3 years of treatment | 20,0% | 12,5% | 39% (27–49), p≤0.001 |
Analysis of pooled data from the SOTI and TROPOS studies showed that in patients (over 80 years of age at study entry), Bivalos reduced the relative risk of developing new vertebral fractures by 32% over the 3-year study period (fracture rate with placebo was 26.5%). , and when taking strontium ranelate - 19.1%). Among patients without a history of fractures, but with at least one additional risk factor for fractures (n = 176), in whom pre-treatment BMD values of the lumbar spine and/or femoral neck were in the range characteristic of osteopenia, use of the drug Bivalos for 3 years of treatment reduced the risk of developing a first vertebral fracture by 72% (the incidence of vertebral fracture with strontium ranelate was 3.6%, and with placebo - 12.0%). Following the TROPOS study, data were analyzed in the subgroup of patients over 74 years of age at high risk of fracture—femoral neck BMD T-score ≤ –3 SD (the range used in the study was –2.4 SD according to NHANES III). In this group (n=1977, i.e. 40% of patients who took part in the TROPOS study), Bivalos reduced the risk of hip fractures by 36% over 3 years of treatment compared with placebo (Table 2). Table 2 Proportion of patients with femoral fractures and reduced relative risk among patients with BMD ≤–2.4 SD (NHANES III) aged ≥74 years
TROPOS Study | Placebo | Bivalos | Relative risk reduction compared with placebo (95% confidence interval), p value |
n=995 | n=982 | ||
| Fracture of the femur during 3 years of treatment | 6,4% | 4,3% | 36% (0–59), p=0.046 |
Buy Bivalos powder for oral suspension 2g No. 28 in pharmacies
Bivalos Buy Bivalos in pharmacies DOSAGE FORMS powder for oral suspension 2g
MANUFACTURERS Servier Laboratories (France)
GROUP Drugs for the treatment of osteoporosis
COMPOSITION The active substance is strontium ranelate. 1 sachet contains 2 g of the active substance. Excipients: aspartame (E951), maltodextrin, mannitol.
INTERNATIONAL NON-PROPENTED NAME Strontium ranelate
PHARMACOLOGICAL ACTION Bivalos is a non-hormonal drug for the treatment of osteoporosis in postmenopausal women. Stimulates the formation and inhibits bone resorption, which leads to normalization of bone structure and, as a result, reducing the risk of vertebral and femoral neck fractures. Strontium ranelate does not change the crystallization characteristics of bone tissue. The combined effects of strontium distribution in bone tissue and increased radiographic absorption of strontium relative to calcium lead to increased bone mineral density (BMD). The medicinal formula of strontium ranelate contains two atoms of stable strontium and one molecule of ranelic acid, as well as an organic part, due to which the required molecular weight values are achieved, favorable pharmacokinetic properties and good tolerability of the drug are ensured. Absorption, distribution and binding of ranelic acid to plasma proteins are quite low, due to the high polarity of the molecule. Ranelic acid does not accumulate and does not exhibit metabolic activity in animals and humans. Absorbed ranelic acid is quickly and unchanged excreted from the human body through the kidneys. The absolute bioavailability of strontium after oral administration of 2 g of strontium ranelate is 25%. Maximum plasma concentrations are achieved 3-5 hours after a single dose of 2 g of the drug. A steady state is achieved after 2 weeks of therapy. Being a divalent cation, strontium is not metabolized in the human body. Strontium ranelate does not inhibit enzymes of the cytochrome P450 system. The effective half-life of strontium is approximately 60 hours. Strontium is excreted through the kidneys, gastrointestinal tract and breast milk.
INDICATIONS FOR USE Treatment of osteoporosis in postmenopausal women to reduce the risk of vertebral and femoral neck fractures.
CONTRAINDICATIONS Hypersensitivity to strontium ranelate or any other component of the drug. Pregnancy and breastfeeding: the drug is intended only for the treatment of postmenopausal women. Strontium ranelate should not be prescribed during pregnancy and lactation. Use in children and adolescents, patients in this age group is not recommended.
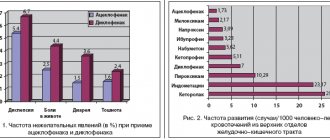
SIDE EFFECTS General: nausea, diarrhea, headache and skin irritation. These effects are mild, short-term in nature and do not require discontinuation of the drug.
INTERACTIONS Milk and dairy products, as well as medications containing calcium, may reduce the bioavailability of strontium ranelate by approximately 60-70%. The simultaneous use of antacid drugs and the drug Bivalos is allowed. When prescribing antibiotics from the group of tetracyclines or quinolones, treatment with Bivalos should be suspended. When combined with vitamin D, no interactions were found. When combining Bivalos with non-steroidal anti-inflammatory drugs (including acetylsalicylic acid), anilides (such as paracetamol), H2 blockers, proton pump inhibitors, diuretics, digoxin and cardiac glycosides, organic nitrates and other vasodilators used for heart disease, calcium channel blockers, beta-blockers, ACE inhibitors, angiotensin receptor antagonists, selective beta2-agonists, oral anticoagulants, platelet aggregation inhibitors, statins, fibrates and benzodiazepine derivatives, no interaction was established.
METHOD OF APPLICATION AND DOSAGE The drug should be taken orally only in the form of a suspension obtained after stirring the powder in a glass of water. The recommended dose is 2 g (contents of one sachet) per day. Due to the chronic nature of the disease, Bivalos is intended to be taken for a long time. Due to the fact that milk and dairy products can reduce the absorption of strontium ranelate, it is necessary to take the drug between meals, preferably before bedtime, at least 2 hours after eating milk, dairy products and/or dietary supplements or calcium supplements . The prepared suspension should be consumed orally immediately after preparation. If the dietary intake of calcium and vitamin D is insufficient, patients taking Bivalos must additionally be prescribed these substances in the form of nutritional supplements. In elderly patients, no dose adjustment is required. In patients with mild or moderate renal failure, no dose adjustment is required. Since strontium ranelate is not metabolized in the body, no dose changes are required in patients with liver failure.
SPECIAL INSTRUCTIONS Due to the lack of data on the safety of the use of strontium ranelate in patients with severe renal failure, the drug is not recommended for use in patients with creatinine clearance less than 30 ml/min. Bivalos should be used with caution in patients at high risk of thromboembolism (VTE), including patients with a history of episodes of VTE. The presence of the excipient aspartame in Bivalos may cause an undesirable reaction in patients with phenylketonuria. Strontium ranelate affects the results of colorimetric methods for assessing calcium levels in blood and urine. Bivalos does not affect the ability to drive vehicles or perform work that requires an increased speed of psychophysical reactions.
STORAGE CONDITIONS List B. Keep out of the reach of children.
Use of the drug Bivalos
For oral use. The recommended daily dose is 2 g of strontium ranelate (contents of 1 single-dose package) per day; before use, the contents of the package are dissolved in a glass of water. Bivalos should be taken between meals. It is recommended to take Bivalos before bed, preferably no earlier than 2 hours after eating. The contents of one sachet should be poured into a glass, add water and stir the granules in the water until a suspension is formed (uniform distribution of the granules in the water). The suspension must be drunk immediately after preparation. The shelf life of the prepared suspension is 24 hours. If the suspension cannot be drunk immediately after preparation, it should be stirred before taking. Bivalos is intended for long-term use.
Side effects of the drug Bivalos
The drug Bivalos was studied in clinical studies that involved about 8,000 patients. Long-term safety of the drug was assessed in postmenopausal women with osteoporosis receiving strontium ranelate 2 g/day for 60 months (n=3352) or placebo (n=3317) during clinical trials. The average age at enrollment was 75 years, and 23% of patients included in the study were aged 80–100 years. The overall incidence of side effects with strontium ranelate did not differ from that with placebo, and side effects were generally mild and reversible. The most commonly reported side effects were nausea and diarrhea, usually at the beginning of treatment, after which no significant differences were noted between the compared groups. Discontinuation of therapy was mainly due to nausea (1.3 and 2.2% in the placebo and strontium ranelate groups, respectively). The following are side effects that could be associated with the use of strontium ranelate according to the frequency of their development: very often (1/10); often (1/100, but ≤1/10); uncommon (1/1000 and ≤1/100); rare (1/10,000 and ≤1/1000); very rare (≤1/10,000). From the nervous system : often: headache, impaired consciousness, memory. Uncommon: convulsions. From the gastrointestinal tract Often: nausea, diarrhea, loose stools. Frequency not known: vomiting, abdominal pain, irritation of the oral mucosa, including stomatitis and/or ulcerative lesions of the oral mucosa. From the musculoskeletal system Frequency not known: musculoskeletal pain, including muscle spasm, myalgia, bone pain, arthralgia and pain in the extremities. From the skin and subcutaneous tissue Frequency is not known: manifestations of hypersensitivity reactions from the skin, including rash, itching, urticaria, angioedema, Stevens-Johnson syndrome; cases of severe hypersensitivity reactions - drug rash accompanied by eosinophilia and systemic symptoms (DRESS syndrome) (see section SPECIAL INSTRUCTIONS). Common: dermatitis, eczema. If serious allergic reactions develop, treatment with Bivalos should be discontinued. Uncommon: venous thromboembolism. Clinical studies lasting more than 4 years showed that the annual incidence of venous thromboembolism with strontium ranelate compared with placebo was about 0.7% with a relative risk of 1.42 (confidence interval 1.02, 1.98, p = 0.036 ). Laboratory data There was a reversible increase in activity (3 times the upper limit of normal) of creatine kinase (its musculoskeletal fraction) in the group of individuals who took strontium ranelate (1.4%) compared with placebo (0.6%). In most cases, these indicators spontaneously normalized without changing the treatment regimen.
Bivalos por d/prig suspension d/oral sachet 2g No. 28
Description
Powder from white to pale yellow.
When the powder is dissolved in water, an opaque white suspension is formed.
The medicinal formula of strontium ranelate contains two atoms of stable strontium and one molecule of ranelic acid (the organic part, thanks to which the required molecular weight values are achieved, favorable pharmacokinetic properties and good tolerability of the drug are ensured). The pharmacokinetics of strontium and ranelic acid were assessed in a group of healthy young men and healthy postmenopausal women, as well as during long-term use of the drug in a group of postmenopausal women with osteoporosis, including elderly women.
Absorption, distribution and binding of ranelic acid to plasma proteins are quite low, due to the high polarity of the molecule.
Ranelic acid does not accumulate and does not exhibit metabolic activity in animals and humans.
Absorbed ranelic acid is quickly and unchanged excreted from the human body by the kidneys.
Absorption
The absolute bioavailability of strontium after oral administration of 2 g of strontium ranelate is approximately 25% (19–27%). Cmax in blood plasma is achieved 3–5 hours after a single dose of 2 g of the drug. Css is achieved after 2 weeks of therapy. Taking strontium ranelate with food, dietary supplements and calcium supplements reduces the bioavailability of strontium by approximately 60–70% compared to the bioavailability level when the drug is taken 3 hours after a meal. Given the relatively slow absorption of strontium, calcium intake, medications and dietary supplements should be avoided both before and after taking Bivalos®. Vitamin D medications and dietary supplements do not have any effect on strontium absorption.
Distribution
Vd of strontium - approximately 1 l/kg. The binding of strontium to human plasma proteins is low (25%), while strontium has a high affinity for bone tissue. Measurement of strontium concentration in iliac bone biopsies of patients receiving strontium ranelate at a dose of 2 g/day for a long time (up to 60 months) indicates that strontium concentration in bone tissue reaches a plateau after approximately 3 years of therapy. There are no data on the elimination of strontium from bone tissue after cessation of therapy.
Biotransformation
Being a divalent cation, strontium is not metabolized in the human body. Strontium ranelate does not inhibit enzymes of the cytochrome P450 system.
Elimination
Elimination of strontium is time- and dose-dependent. The effective T1/2 of strontium is approximately 60 hours. Strontium is excreted by the kidneys and through the gastrointestinal tract. Plasma clearance of strontium is approximately 12 ml/min (CV 22%), and renal clearance is approximately 7 ml/min (CV 28%).
Pharmacokinetics in special groups of patients
Elderly patients. Pharmacokinetic data in this target population indicate no relationship between age and measured strontium clearance.
Patients with renal failure. In patients with mild to moderate renal impairment (Cl creatinine 30–70 ml/min), strontium clearance decreases as creatinine Cl decreases (by approximately 30% for creatinine Cl values ranging from 30 to 70 ml/min), resulting in an increase in plasma strontium concentration. In clinical studies, 85% of patients had creatinine Cl between 30 and 70 ml/min, and 6% had <30 ml/min at enrollment, with an average creatinine Cl of approximately 50 ml/min. Thus, dose adjustment of the drug is not required in patients with mild to moderate renal failure. There are no data on the pharmacokinetics of the drug in patients with severe renal failure (Cl creatinine <30 ml/min).
Patients with liver failure. There are no data on the pharmacokinetics of the drug in patients with liver failure. However, given the pharmacokinetic properties of strontium, it can be assumed that they do not change in this group of patients.
In in vitro studies, strontium ranelate stimulates bone formation in bone tissue culture, and also stimulates the replication of osteoblast precursors and collagen synthesis in bone cell culture; reduces bone resorption by suppressing the differentiation of osteoclasts, as well as their resorptive activity.
As a result of the action of the drug, the balance between the formation and destruction of bone tissue changes towards the processes of bone formation.
The activity of strontium ranelate has been studied experimentally using various preclinical models. In particular, in experiments on intact rats, the use of strontium ranelate led to an increase in trabecular bone mass, the number of trabeculae and their thickness, resulting in improved mechanical properties of the bone.
In the bone tissue of humans and experimental animals to which the drug was prescribed, strontium ranelate was mainly absorbed on the surface of hydroxyapatite crystals and only to a small extent replaced calcium in these crystals in the newly formed bone tissue. Strontium ranelate does not change the characteristics of bone tissue crystals. According to iliac crest biopsies performed after treatment with strontium ranelate at a dose of 2 g/day for up to 60 months in clinical studies, no adverse effects on bone quality or mineralization were established.
The combined effects of strontium distribution in bone tissue (see Pharmacokinetics) and increased x-ray absorption by strontium relative to calcium result in increased bone mineral density (BMD), as measured by two-photon x-ray absorptiometry. Data obtained to date indicate that these factors account for approximately 50% of the increase in BMD after 3 years of treatment with Bivalos® at a dose of 2 g/day. This feature should be taken into account when interpreting changes in BMD during treatment with Bivalos®.
In studies confirming the ability of Bivalos® to reduce the risk of fractures, the measured mean BMD value increased in the group of patients receiving Bivalos® compared to the baseline value - for the lumbar vertebrae by approximately 4% per year, and for the femoral neck by 2% in year; after 3 years, the increase in BMD was 13–15% and 5–6%, respectively, according to various studies.
Starting from the third month of therapy and during 3 years of observation, there was an increase in the indicators of biochemical markers of bone tissue formation (bone fraction of ALP and C-terminal propeptide of type I procollagen) and a decrease in indicators of markers of bone tissue resorption (cross-linked C-terminal and N-terminal telopeptides in urine) compared to placebo.
For strontium ranelate, a secondary effect in relation to the main pharmacological properties is a slight decrease in serum concentrations of calcium and parathyroid hormone, as well as an increase in the concentration of phosphorus in the blood and the activity of total alkaline phosphatase, which, however, is not accompanied by any clinical effects.
Risk factors for postmenopausal osteoporosis include low bone mass, decreased BMD, early menopause, a history of smoking, and a family history of osteoporosis.
One of the most clinically significant complications of osteoporosis is the development of fractures, and the risk of fractures increases with the number of risk factors.
Treatment of postmenopausal osteoporosis
In studies involving more than 6.5 thousand postmenopausal women with documented osteoporosis, the effect of Bivalos® on the prevention of fractures was studied.
It was shown that the use of Bivalos® reduced the relative risk of new vertebral fractures by 41% after 3 years of therapy. This effect became significant starting from the first year of therapy. The relative risk of vertebral fractures accompanied by clinical manifestations (defined as fractures with the development of pain and/or a decrease in the patient's height by at least 1 cm) decreased by 38%. Also, treatment with Bivalos® compared to placebo significantly reduced the number of patients whose height decreased by 1 cm or more. The beneficial effects of Bivalos® compared to placebo were also demonstrated when assessing quality of life using the special QUALIOST® scale and general perception of health using the general SF-36 scale.
The effectiveness of the use of the drug Bivalos® has been confirmed in reducing the risk of new vertebral fractures, incl. and in patients with no history of fractures associated with osteoporosis.
A retrospective analysis showed that in patients with no history of fractures and BMD of the lumbar vertebrae and/or femoral neck indicating osteopenia, the use of Bivalos® for 3 years reduced the risk of a first vertebral fracture by 72%.
In the group of patients with a high risk of fractures (T-score of the femoral neck BMD index within ≤3 SD) over the age of 74 years, taking Bivalos® for 3 years reduced the risk of femoral fractures by 36% compared with the group of patients those receiving placebo.
Treatment of osteoporosis in men
The effectiveness of Bivalos® for the treatment of osteoporosis in men was demonstrated during a 2-year clinical study involving 243 patients at high risk of fractures (the average age of patients was 72.7 years, the average T-score for BMD of the lumbar spine was 2 .6; 28% with a history of vertebral fractures).
During the study, patients received calcium (1000 mg/day) and vitamin D (800 IU/day).
A statistically significant increase in BMD was observed 6 months after the start of therapy (compared to placebo).
After 12 months of therapy with Bivalos®, a statistically significant increase in the average BMD of the lumbar spine was shown (the main criterion for effectiveness was 5.32%; p <0.001), similar values were noted in studies examining the effect of Bivalos® on the prevention of fractures in postmenopausal women .
A statistically significant increase in femoral neck BMD and femoral BMD index (p <0.001) was observed 12 months after the start of therapy with Bivalos®.
In patients with chronic renal failure, it is recommended to monitor renal function. If severe renal failure develops, the question of continuing treatment with Bivalos® should be decided on an individual basis.
In clinical studies, an increase in the incidence of VTE was noted, incl. pulmonary embolism (see “Side effects”). The cause of this phenomenon has not yet been established. When treating patients at risk of VTE or patients with a possible increased risk of VTE, special attention should be paid to identifying possible symptoms of this complication, as well as carrying out its adequate prevention. It should be borne in mind that the risk of venous thrombosis is increased in patients on bed rest and/or in preparation for surgery.
Strontium affects the results of colorimetric methods for assessing calcium levels in blood and urine. In this regard, methods such as inductively coupled plasma atomic emission spectrometry or atomic absorption spectrometry should be used to more accurately estimate calcium concentrations in blood and urine.
The presence of the excipient aspartame in Bivalos® may cause an undesirable reaction in patients with phenylketonuria (a rare metabolic disorder).
Treatment with Bivalos® should be discontinued if severe allergic reactions develop.
During the use of the drug Bivalos®, cases of severe, in some cases fatal, hypersensitivity reactions, incl. drug rash in combination with eosinophilia and systemic symptoms (DRESS syndrome) (see “Side effects”). DRESS syndrome presents with rash, fever, eosinophilia, and systemic symptoms (such as adenopathy, hepatitis, interstitial nephropathy, interstitial lung disease). The time from the start of taking Bivalos® to the development of this side effect was usually 3–6 weeks. In most cases, DRESS syndrome resolved after discontinuation of the drug and initiation of GCS therapy. The process of resolving this side effect could be lengthy. There have been cases of relapse of DRESS syndrome when GCS was discontinued.
Patients should be informed that if a rash appears, they should immediately stop taking Bivalos®, do not resume therapy, and consult a doctor. Patients who have stopped taking Bivalos® due to the development of hypersensitivity reactions should not resume therapy with this drug.
Impact on the ability to drive vehicles and perform work that requires an increased speed of psychophysical reactions
Does not affect.
Compound
Powder for the preparation of suspension for oral administration 1 sachet strontium ranelate 2 g excipients: aspartame, maltodextrin, mannitol
in sachet 2 g; in a cardboard pack there are 7, 14, 28, 56, 84 or 100 sachets.
Application
treatment of osteoporosis in postmenopausal women to reduce the risk of vertebral and femur fractures (including femoral neck fractures);
treatment of osteoporosis in men to reduce the risk of fractures.
Bivalos® is intended only for the treatment of postmenopausal women.
There are no clinical data on the use of strontium ranelate during pregnancy.
There were no effects of Bivalos® on reproductive function in animal studies.
In animal experiments, the administration of strontium ranelate in high doses during pregnancy led to the development of reversible bone deformities in the offspring.
If pregnancy occurs while taking Bivalos®, treatment should be stopped immediately.
Strontium is excreted in breast milk. Bivalos® should not be prescribed to women who are breastfeeding.
The safety of Bivalos® was studied in clinical studies involving approximately 8,000 patients.
The safety of the drug was confirmed during a study involving women with postmenopausal osteoporosis who took long-term (treatment duration reached 60 months) strontium ranelate at a dose of 2 g/day, the average age of patients at the time of inclusion in the study was 75 years, 23% of patients were aged from 80 to 100 years.
The overall frequency of adverse reactions when prescribing strontium ranelate did not differ significantly from that in the group of patients receiving placebo, while adverse reactions to the drug were usually mild and short-lived. The most common adverse events were nausea and diarrhea, which were mainly observed at the beginning of therapy, and subsequently the incidence of these adverse reactions was not significantly different between the placebo and strontium ranelate groups.
The following is a list of adverse reactions noted in clinical studies, the connection of which with the use of strontium ranelate, at least, cannot be excluded. The frequency is presented in comparison with the placebo group in the following gradation: very often (>1/10); often (>1/100, <1/10); uncommon (>1/1000, <1/100); rare (>1/10000, <1/1000); extremely rare (<1/10000) and unknown frequency (frequency cannot be calculated from available data).
From the side of the central nervous system: often - headache, impaired consciousness, memory loss; infrequently - convulsions.
From the circulatory system: often - venous thromboembolism.
From the gastrointestinal tract: often - nausea, diarrhea, loose stools.
From the skin and subcutaneous tissues: often - dermatitis, eczema.
There were no significant differences in the nature of adverse events in the groups of patients younger and older than 80 years at the time of inclusion in the study.
In clinical studies, it was shown that the annual incidence of venous thromboembolic complications in the group of patients receiving strontium ranelate was 0.7% during 5 years of observation with a relative risk of 1.4 (95% CI 1.0; 2.0) according to compared to the placebo group.
Laboratory indicators. Transient acute increases in the concentration of the muscle fraction of creatine phosphokinase (CPK), more than 3 times higher than the ULN, were observed with a frequency of 1.4 and 0.6% in the groups of patients receiving strontium ranelate and placebo, respectively. In most cases, CPK concentrations returned to normal on their own with continued treatment with Bivalos® without changing therapy.
During post-marketing use of the drug, the following side effects were noted.
From the gastrointestinal tract: unknown frequency - vomiting; abdominal pain; damage to the oral mucosa, incl. stomatitis and ulceration of the oral mucosa.
From the skin and subcutaneous tissues: unknown frequency - skin hypersensitivity reactions, incl. rash, skin itching, urticaria, angioedema; severe hypersensitivity reactions, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis and drug rash accompanied by eosinophilia and systemic manifestations (DRESS syndrome) (see “Special Instructions”); alopecia.
From the musculoskeletal system and connective tissue: unknown frequency - muscle spasm, myalgia, bone pain, arthralgia and pain in the extremities.
Mental disorders: unknown frequency - confusion.
From the hepatobiliary system: unknown frequency - increased activity of hepatic transaminases (due to skin hypersensitivity reactions).
General disorders and symptoms: unknown frequency - peripheral edema, hyperthermia (due to skin hypersensitivity reactions).
From the respiratory system: unknown frequency - bronchial hyperreactivity.
From the circulatory and lymphatic system: unknown frequency - bone marrow failure, eosinophilia (due to skin hypersensitivity reactions), lymphadenopathy (due to skin hypersensitivity reactions).
Food products, in particular milk and dairy products, as well as drugs and dietary supplements containing calcium, can reduce the bioavailability of strontium ranelate by approximately 60–70%. In this regard, the administration of strontium ranelate and these substances should be separated by a period of at least 2 hours (see “Pharmacokinetics”).
An in vivo clinical interaction study showed that administration of aluminum and magnesium hydroxides both 2 hours before and concomitantly with strontium ranelate caused a slight decrease in the absorption of strontium ranelate (20–25% reduction in AUC), whereas when administered an antacid of the drug, 2 hours after taking strontium ranelate, the level of absorption remains practically unchanged. Thus, it is preferable to take antacid drugs no earlier than 2 hours after taking strontium ranelate. However, in practice, this drug regimen is inconvenient, since strontium ranelate is recommended to be taken before bedtime. In this regard, simultaneous use of antacids and strontium ranelate is allowed.
Since molecular complexes containing divalent cations interact at the gastrointestinal tract level with antibiotics of the tetracycline and quinolone series, the simultaneous use of strontium ranelate and these drugs leads to a decrease in the absorption of these antibiotics. In this regard, it is not recommended to take these drugs at the same time. In order to prevent such an interaction, when prescribing antibiotics from the group of tetracyclines or quinolones, treatment with strontium ranelate should be suspended.
When strontium ranelate was combined with dietary supplements or vitamin D preparations, no interaction was observed.
No clinically significant interaction or increase in the level of strontium ranelate in the blood was observed when strontium ranelate was combined with the following drugs: NSAIDs (including acetylsalicylic acid), anilides (for example paracetamol), histamine H2 receptor blockers and proton pump inhibitors, diuretics, cardiac glycosides (in including digoxin), organic nitrates and other vasodilators used for heart diseases, CCBs, beta-blockers, ACE inhibitors, angiotensin II receptor antagonists, selective beta2-agonists, oral anticoagulants, platelet aggregation inhibitors, statins, fibrates and benzodiazepine derivatives .
Inside.
The recommended dose is 2 g/day (the contents of one sachet). Due to the chronic nature of the disease, Bivalos® is intended to be taken for a long time.
It is recommended to take the drug before bedtime. You can take a horizontal position immediately after taking the drug.
The drug Bivalos® should be taken in the form of a suspension, for which you need to pour the powder from the sachet into a glass, add water and stir until the powder is evenly distributed in the water.
Although studies have demonstrated the stability of strontium ranelate in suspension over 24 hours, it is recommended that the suspension be ingested immediately after preparation.
Due to the fact that food, drugs and dietary calcium supplements, milk and dairy products can reduce the absorption of strontium ranelate, it is necessary to take the drug between meals, preferably before bedtime, at least 2 hours after eating, consuming milk, dairy products , dietary supplements or calcium preparations (see “Interaction” and “Pharmacokinetics”).
Patients taking Bivalos® should be additionally prescribed medications and/or dietary supplements of calcium and vitamin D if their dietary intake of these substances is insufficient.
Use in elderly patients
No dose adjustment is required depending on age.
The effectiveness and safety of the drug Bivalos® was studied in postmenopausal patients of different ages (the maximum age at inclusion in the study was 100 years).
Use for renal failure
In patients with mild or moderate renal failure (creatinine clearance 30–70 ml/min), no dose adjustment is required (see Pharmacokinetics). In patients with severe renal failure (Cl creatinine <30 ml/min), Bivalos® should be prescribed with caution (see “Pharmacokinetics” and “Special Instructions”).
Use for liver failure
Since strontium ranelate is not metabolized in the body, no dose adjustment is required in patients with liver failure.
Use in children and adolescents
The effectiveness and safety of Bivalos® in children and adolescents has not been studied, and therefore it is not recommended to prescribe this drug to patients in this age group.
When studying the use of strontium ranelate at a dose of 4 g/day for 25 days in a group of healthy postmenopausal women, good tolerability of the drug was demonstrated.
In cases of drug overdose during clinical trials (up to 4 g/day with a maximum duration of 147 days), no clinically significant side effects were observed.
In order to reduce the absorption of the active substance in the gastrointestinal tract, it is recommended to take milk or antacids.
If the recommended dose is significantly exceeded, vomiting must be induced to remove the unabsorbed active substance.
known hypersensitivity to strontium ranelate and/or any of the components of the drug;
children under 18 years of age (due to lack of data on use).
With caution: in patients with severe renal failure (Cl creatinine <30 ml/min); in patients with an increased risk of developing venous thromboembolism (VTE), incl. with a history of VTE episodes.
Possible product names
- Bivalos por d/prig suspension d/oral sachet 2g No. 28
- BIVALOS 2.0 P-K D/PRIG. SUSPENSIONS No. 28
- BIVALOS 2.0 N28 POR D/SUSP
- BIVALOS POR. D/SUSP.ORAL. SACHE 2 G. X28
- BIVALOS POR. COOK. SUSP D/VN. TAKING SACHE 2G No. 28
- BIVALOS 2G POR. D/PRIG. SUSP. D/ORAL SACHE X28 (R)
- (Bivalos) Bivalos por d/prig suspension for oral administration sachet 2g No. 28
Special instructions for the use of the drug Bivalos
Patients receiving treatment with strontium ranelate are advised to take vitamin D and calcium supplements if dietary intake is insufficient. Consumption of food, including milk and dairy products, and medications containing calcium, may reduce the absorption and bioavailability of strontium ranelate by approximately 60–70%. Therefore, the interval between taking Bivalos and these substances should be at least 2 hours. Given the slow absorption of strontium ranelate, it is recommended to take Bivalos before bed, preferably no earlier than 2 hours after meals. The effectiveness and safety of strontium ranelate have been established in women of different age categories (up to the age of 100 years at the time of inclusion in the study) in the postmenopausal period with osteoporosis. There is no need for dose adjustment depending on age. Patients with mild to moderate renal failure (creatinine clearance 30–70 ml/min) do not require dose adjustment. Strontium ranelate is not recommended for use in patients with severe renal impairment (creatinine clearance ≤30 ml/min) due to lack of data. In patients with chronic renal failure, periodic assessment of renal function is recommended. The question of continuing therapy with Bivalos in patients with severe renal failure is decided individually. Strontium ranelate is not metabolized, so patients with impaired liver function do not need dose adjustment. The effectiveness and safety of strontium ranelate in children and adolescents has not been established, therefore its use in these age groups is not recommended. Clinical studies have shown that strontium ranelate therapy was associated with an increased annual incidence of venous thromboembolism, including pulmonary embolism. The reason for this has not been established. Bivalos should be used with caution in patients with an increased risk of developing venous thromboembolism, including patients with a history of this pathology. When treating patients at risk, special attention should be paid to identifying possible signs and symptoms of venous thromboembolism and appropriate prevention. Strontium can affect the results of determining the concentration of calcium in the blood and urine using the colorimetric method. Therefore, to accurately assess the concentration of calcium in the blood and urine in clinical practice, it is recommended to use the inductively coupled plasma atomic emission spectrometry method or the atomic absorption spectrometry method. Bivalos contains aspartame, which is a source of phenylalanine, which is contraindicated in patients with phenylketonuria. During use of the drug, it is possible to develop a severe hypersensitivity reaction - a drug rash, which is accompanied by eosinophilia and systemic symptoms (DRESS syndrome). (See SIDE EFFECTS section). DRESS syndrome is characterized by rash, fever, eosinophilia, and systemic symptoms (adenopathy, hepatitis, interstitial nephropathy, and interstitial lung disease). Most often, DRESS syndrome develops 3–6 weeks after starting the drug. After stopping the drug and starting GCS therapy, the prognosis is favorable. Recovery may be gradual. After discontinuation of GCS therapy, symptoms may resume. Patients should be warned about the need to immediately stop taking the drug and consult a doctor if a rash occurs while taking Bivalos. Patients who have stopped treatment with the drug due to hypersensitivity reactions should in no case resume taking it in the future. The drug Bivalos is intended only for use in postmenopausal women. There are no clinical data on the use of strontium ranelate during pregnancy. Experimental studies have shown that in high doses it can cause reversible changes in bone tissue in the offspring of rats and rabbits that were given the drug during pregnancy. If pregnancy occurs, Bivalos should be discontinued. Strontium is excreted in breast milk, so it is not recommended to administer strontium ranelate during breastfeeding. Strontium ranelate does not affect the ability to drive vehicles or operate machinery. If signs of side effects appear, caution must be exercised.
Bivalos instructions for use
Pharmacological action The active substance - strontium ranelate - has a dual effect on bone tissue, changing the ratio of metabolic processes in it in favor of osteogenesis. The drug stimulates the division of osteoblasts, increases collagen synthesis, suppresses the synthesis of osteoclasts, which reduces the possibility of bone resorption. Studies found that strontium ranelate increased the number of trabeculae and their thickness, which resulted in increased bone mass and bone strength. A study of bone biopsies up to 60 months after treatment did not show any adverse effects on bone mineralization or changes in bone crystal characteristics. Bone mineral density (BMD) during treatment with Bivalos increases annually by approximately 4% in the lumbar spine and by 2% in the femoral neck; after 3 years, BMD increases by 14% and 6%, respectively. Studies have shown a consistent increase in markers of bone formation (alkaline phosphatase bone-specific fraction and C-terminal propeptide of procollagen type I) and a decrease in the concentration of markers of bone resorption (C-telopeptide in serum and N-telopeptide in urine). Other changes in the biochemical composition of the blood were also recorded: a decrease in the level of Ca and PTH (parathyroid hormone), an increase in the level of P and alkaline phosphatase (alkaline phosphatase), but they are clinically insignificant. The active substance of the drug Bivalos consists of strontium and ranelic acid. Strontium is deposited on apatite crystals, providing clinical effects, and ranelic acid improves the pharmacokinetics and tolerability of the drug. There is no evidence that the acid can accumulate in tissues or biotransform in the body. Ranelic acid is excreted unchanged by the kidneys. The concentration of strontium in the blood is proportional to the dose taken and the time of administration. The half-life of strontium is about 60 hours; strontium is excreted by the kidneys and liver, almost equally. The time of strontium excretion does not depend on the age of the patient and the presence or absence of liver pathology. The maximum concentration of the drug Bivalos is achieved 3-5 hours after a single dose. Taking Bivalos simultaneously with food and/or calcium salts reduces the bioavailability of the drug. Indications for use Treatment of osteoporosis caused by postmenopause, prevention of fractures of the femur and vertebral bodies.
Method of administration The drug Bivalos is used in a dose of 2 g (one sachet) once a day, before use, the contents of the sachet should be dissolved in a glass of water until a homogeneous suspension is formed, taken immediately after preparation, if it was not possible to drink the suspension immediately, it can be stored for 24 hours after cooking. Do not use simultaneously with meals; it is advisable to use the drug shortly before bedtime.
Side effects As a rule, the drug is well tolerated, both with short-term therapy and with long-term use, side effects are usually absent or mild, the frequency of their occurrence is comparable to the frequency when taking placebo. The most common side effects were nausea and/or diarrhea, which were short-term reversible and were usually recorded only at the beginning of treatment. Less commonly observed side effects were headache, memory impairment, convulsions, vomiting, abdominal pain, changes in the condition of the oral mucosa (including stomatitis and ulcerative lesions), myalgia, bone pain, arthralgia, allergic reactions (up to the development of the syndrome Stevens-Johnson), thromboembolism, possible spontaneous increase in creatinine kinase levels. If a severe allergic reaction occurs, Bivalos should be stopped immediately.
Contraindications A contraindication to the use of the drug Bivalos is intolerance to the active substance or any of the components of the drug.
Pregnancy The drug Bivalos is intended for use by postmenopausal women. There are no data on the safety of using the drug during pregnancy. Animal studies have demonstrated the possibility of adverse effects of the drug in non-offspring. If pregnancy occurs, Bivalos should be discontinued.
Drug interactions The combination of Bivalos with calcium preparations leads to a decrease in the bioavailability of Bivalos by approximately 60-70%, therefore, if it is necessary to use these drugs, it is necessary to maintain an interval between their doses of at least 2 hours. Taking non-absorbable antacids based on magnesium and/or aluminum reduces the absorption of Bivalos, so the interval between taking these drugs should be at least 2 hours. Bivalos can impair the absorption of quinolones and tetracycline antibiotics by forming a stable compound with them, so Bivalos should be discontinued during treatment with these drugs. Taking vitamin D, NSAIDs, H2-histamine receptor antagonists, proton pump inhibitors, diuretics, nitrates, antihypertensives, antiplatelet agents, anticoagulants, statins and fibrates does not affect the absorption of Bivalos.
Overdose In experimental studies, even five times the recommended dosage did not lead to the development of any undesirable consequences. If the dose of Bivalos is significantly exceeded, gastric lavage and antacids are recommended.
Release form Granules for preparing a suspension in a sachet 2 g No. 7; Granules for preparing a suspension in a sachet 2 g No. 28; Granules for preparing a suspension in a sachet 2 g No. 56.
Storage conditions Keep out of reach of children, store at room temperature (up to 27 degrees Celsius), avoid direct sunlight.
Composition Active substance: strontium ranelate 2 g. Additional substances: mannitol, aspartame, maltodextrin.
Pharmacological group Medicines used to correct disorders that occur during menopause
Active ingredient: strontium ranelate
Additionally If the patient is unable to obtain sufficient amounts of calcium and vitamin D from food, additional intake is recommended. Taking calcium supplements, as well as milk and dairy products, can significantly reduce the bioavailability of calcium, therefore, an interval of at least 2 hours must be maintained between their use and taking Bivalos, and the same break is necessary after eating food. Taking the drug Bivalos is effective and safe for women of various age groups, which has been proven by numerous studies, so dose adjustment is not required in patients of older age groups. There is no data on the safety of using the drug Bivalos in children and adolescents, therefore the drug is not used in these age groups. In the presence of mild to moderate chronic renal failure, Bivalos can be used in the usual dosage, constantly monitoring the creatinine level; in severe chronic renal failure (creatinine clearance ≤30 ml/min), the use of the drug is not recommended. The drug Bivalos does not undergo biotransformation in the body, so it can be used in patients with impaired liver function. Studies have established a connection between an increase in cases of venous thromboembolism and taking the drug Bivalos, which requires caution when prescribing the drug to patients at risk for this disease and those who have a history of episodes of embolism. Among the components of the drug Bivalos is aspartame, which must be taken into account when prescribing to patients with phenylketonuria. When taking the drug Bivalos, it is possible to develop symptoms of hypersensitivity, including severe ones, such as DRESS syndrome (the appearance of rash, eosinophilia, adenopathy, nephropathy, lung damage). The development of DRESS syndrome is more often observed after 1-1.5 months of taking the drug. With immediate discontinuation of the drug and the use of glucocorticosteroids, all changes are reversible, the prognosis is favorable. The drug is not used in women during pregnancy and lactation, due to the lack of data on the safety of such use. Bivalos does not affect the ability to control complex mechanisms and does not affect the speed of mental reactions.
Interactions of the drug Bivalos
Taking medications containing calcium may reduce the bioavailability of strontium ranelate by approximately 60–70%. Therefore, an interval of at least 2 hours must be maintained between taking Bivalos and the above-mentioned medications. The use of aluminum and magnesium hydroxide simultaneously or less than 2 hours before taking strontium ranelate leads to a slight decrease in the absorption of strontium ranelate (20–25% reduction in AUC). The use of antacids 2 hours after taking strontium ranelate has virtually no effect on the absorption of strontium ranelate, therefore antacids containing aluminum and magnesium hydroxide are recommended to be taken at least 2 hours after taking Bivalos. However, if it is not possible to adhere to taking antacid medications at least 2 hours after taking Bivalos due to the recommended use of Bivalos before bedtime, then simultaneous use of Bivalos and antacid medications is allowed. Divalent cations of strontium ranelate can form complexes in the gastrointestinal tract with oral tetracycline and oral quinolone antibacterial drugs and reduce their absorption. Concomitant use of strontium ranelate with these medications is not recommended. During the use of tetracyclines or quinolones for oral use, treatment with Bivalos should be temporarily discontinued. No interactions have been observed with oral vitamin D preparations. Clinical studies have not revealed any interaction or increase in the level of strontium in the blood when used with drugs commonly used in this group of patients simultaneously with the drug Bivalos. These drugs include: NSAIDs (including acetylsalicylic acid), anilides (for example paracetamol), H2 receptor antagonists and proton pump inhibitors, diuretics, digoxin and cardiac glycosides, organic nitrates, calcium channel blockers, β-adrenergic blockers, ACE inhibitors, antagonists angiotensin II, selective β2-adrenergic receptor agonists, oral anticoagulants, antiplatelet agents, statins, fibrates and benzodiazepine derivatives.
Overdose of the drug Bivalos
In a clinical study examining the effect of repeated administration of strontium ranelate to healthy postmenopausal women at a dose of 4 g/day for more than 25 days, the drug was well tolerated. A single use of strontium ranelate in a dose of up to 11 g in volunteers (healthy young men) did not cause the development of any clinically significant side effects. After overdose episodes during clinical studies (up to 4 g/day, with a maximum treatment duration of 147 days), no clinically significant side effects were observed. In order to reduce the absorption of the active substance, it is advisable to take milk or antacids. In case of significant overdose, gastric lavage is recommended.