- What is viral hepatitis C
- Prevalence of viral hepatitis C
- How do you get infected with the hepatitis C virus?
- Hepatitis C virus testing
- What is special about the hepatitis C virus?
- How does hepatitis C occur?
- When do signs and symptoms of hepatitis C appear?
- What period after infection must pass for laboratory markers of viral hepatitis C to appear in tests?
- Can viral hepatitis C be cured?
- Where to start treatment?
- Which doctors treat hepatitis C?
- When to start treatment?
- Is it possible not to treat viral hepatitis C?
- Modern methods of treating viral hepatitis C
- Hepatitis S/C (Video - interview with B.L. Lurie)
What is viral hepatitis C, how does it manifest, why is it dangerous?
How is the virus transmitted and can hepatitis C be cured? Which doctors treat and where to start treatment? Hepatitis C
(
Hepatitis C Virus
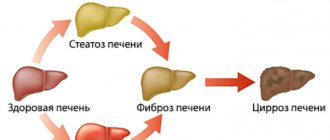
) is an inflammatory liver disease caused by the HCV virus (Hepatitis C Virus). The disease can occur in both acute and chronic forms. Chronic viral hepatitis C, for many years, occurs without symptoms, which makes the disease especially dangerous. The HCV virus uses healthy liver cells to replicate, causing the cells to be destroyed and replaced with fibrous tissue. Without treatment for hepatitis C, the likelihood of developing the worst consequences, such as cirrhosis and liver cancer, increases dramatically. That is why it is so important to promptly identify the disease and begin therapy. Modern antiviral drugs can cure hepatitis C completely.
Symptoms of hepatitis C
The disease is most often asymptomatic, but some nonspecific manifestations are possible:
- fatigue, increased fatigue;
- nausea, loss of appetite.
- heaviness in the right hypochondrium;
- joint pain;
- sleep disorders;
- skin itching.
up
How do you get infected with the hepatitis C virus?
The virus is transmitted through blood. You can become infected with the virus through tattooing, piercing, visiting a manicure salon, medical manipulations with blood, including blood transfusions, administering blood products, operations, or at a dentist appointment. Infection is also possible through shared use of toothbrushes, razors, and manicure accessories. Read more…
Sexual transmission is rare, as is transmission of the virus from the mother during pregnancy. Breastfeeding is not prohibited if you have hepatitis C, but you should be careful if blood appears on your nipples.
It is impossible to become infected with the hepatitis C virus through household contacts. The virus is not transmitted by airborne droplets, shaking hands, hugging or using shared utensils. Patients with viral hepatitis C do not need isolation and do not pose a danger to others. In Russia, however, they are exempt from military conscription.
up
How I received treatment
And so it began. One tablet per day, preferably at the same time, with plenty of water. According to the instructions, side effects could appear in the first week of treatment: headache, rash and itching, fatigue and a few other non-serious symptoms. But according to my doctor, none of her patients have encountered them.
And I didn’t feel anything at all. I swallowed the pill like a piece of chalk, following a signal on my phone, and forgot about the treatment for a day. There were so many new sensations that, closer to the two-week mark, the thought began to itch: “Have they really been bred into a dummy?”
Two weeks later I went for the first control test. There are several of them: after 2, 4, 12 weeks, and then, after the end of treatment, after six months and a year. I took a qualitative PCR test, a test that determines the presence of a virus in a blood sample. It is the fact of presence itself, and not its quantity. I would like to note right away that I took all the control tests at my own expense in commercial laboratories, since I did not officially undergo treatment and there were no grounds for prescribing tests under the compulsory medical insurance contract. Depending on the laboratory and current promotions, I paid from 500 to 700 RUR for each sample.
The doctor explained that the first test, two weeks after the start of treatment, can be either positive or negative. The analysis after four weeks should already be negative, like all subsequent ones. If not, you need to supplement the treatment regimen or interrupt it.
And now my first two weeks are over. I really hoped that I would receive confirmation that everything was not in vain. But the analysis turned out to be positive. And although I knew that the virus could still be detected at the first cutoff, after this result I began to openly worry about success. The next two weeks were the hardest. I still spent hours taking pills, dieting, giving up alcohol completely, and dreading the next test. And finally he waited.
For the first time in 17 years, the test did not detect HCV in my body. My “everything is lost” mood instantly changed to “hurray, we are breaking, the Swedes are bending.” And although I knew that sometimes the virus returns several months after treatment, it was at that moment that I believed that everything would work out for me. I already automatically took the therapy and did not feel any side effects. At the end of the course, the test again did not detect the virus in my blood.
The course ended, and I began to get used to living without hepatitis. I still couldn’t believe that the once incurable disease was gone. And I felt that I had left for good, even though there were still two control tests ahead.
By the first half of the year without hepatitis, I began to feel the effect of the treatment. The bitter taste in my mouth stopped bothering me. The feeling of bloating in the right hypochondrium disappeared, sleep improved, and performance increased. I had one last control test left to say with a clear conscience: I had cured hepatitis C.
This is how my epic with the treatment of hepatitis C ended. The legacy of the disease left antibodies in my blood, which are now always with me. Therefore, now it makes no sense for me to carry out ELISA - an antibody test to diagnose hepatitis - and doctors need to be warned about this. At the same time, despite the presence of antibodies, after treatment, immunity to the virus is not developed, so I can catch this infection in the second round. There are also problems with the gallbladder - cholecystitis has not disappeared. But you can live with it, it can be treated, and you can’t infect anyone with it.
My first positive test after two weeks from the start of treatment
The first “minus” four weeks after the start of treatment
12 weeks from the start of treatment - no virus detected
Six months after treatment: still no virus
A year after treatment - no virus, hurray!
Hepatitis C virus testing
To determine the hepatitis C virus in the blood, it is necessary to take an Anti-HCV antibody test, which shows whether there has ever been contact with the virus. The cost of analysis is 550 rubles
.
- Anti-HCV - negative - no contact
- Anti-HCV - positive - there was contact
The presence of antibodies does not mean the presence of the virus in the blood, and if the Anti-HCV result is positive, a PCR analysis of HCV-RNA is done
, based on the results of which we determine whether the hepatitis C virus is present in the blood.
The cost of the analysis is 750 rubles
.
Sign up for a free consultation to schedule an examination. ANONYMOUSLY.
You can get tested:
- from 9:00 to 17:30 on weekdays
- from 9:00 to 15:00 on Saturday
Sign up by phone
+7
Seven days a week from 9:00 to 21:00
Request a call back
up
How I bought Indian tablets
Now I catch myself thinking that I was lucky several times in this story. I was lucky to meet a competent hepatologist and lucky with the choice of the supplier’s website. After contacting me through the feedback form, my personal manager contacted me. She accompanied me promptly and competently until the very end of the transaction. In general, the organization of sales on our Russian side was a pleasant surprise.
First of all, in response to my application, I was introduced to the entire available range of medications with the active ingredient I needed. Since I had already initially decided with the doctor about the drug, I did not change anything and confirmed my order for three jars of Ladyhep. In response, they sent me payment details and an invoice - that’s what an invoice for payment is called in international commerce.
The intermediary offered five different generics of Harvoni, which varied slightly in price
This is what the payment details sent to me looked like:
Invoice for my order, the total cost of which was $740
With the invoice, I went to my bank and made a currency transfer. Then I sent a scan of the payment notification to the manager
My new generation drug. Source: LadyHep
I had to wait 12 days for confirmation of funds being credited. But the next day after confirmation, the parcel was sent immediately. I received a tracking code and waited another 12 days. In total, 27 days passed from the moment of application on the website to the moment of receipt of the shipment at the EMC point of issue.
I exhaled, decided to wait six days until the beginning of November, so that it would be easier to count the weeks, and start taking pills from the first.
What is special about the hepatitis C virus?
After the virus enters the human body, it enters the liver through the bloodstream, infects liver cells and multiplies there.
The hepatitis C virus is characterized by genetic variability and the ability to mutate. There are 6 main genotypes of the virus and more than 40 subtypes. This is why the virus often manages to “deceive” the immune system, which leads to the development of chronic viral hepatitis C.
Hepatitis C is one of the main reasons leading to liver transplantation, which is why it is better not to delay its treatment.
up
WHO activities
In May 2021, the World Health Assembly adopted the first Global Health Sector Strategy on Viral Hepatitis 2016–2021. It highlights the critical role of universal health coverage and aligns the strategy's objectives with those of the Sustainable Development Goals. The main goal of the strategy is to eliminate viral hepatitis as a public health problem. This is reflected in global targets to reduce the number of new cases of viral hepatitis infection by 90% and mortality from viral hepatitis by 65% by 2030. The strategy sets out the measures that countries and the WHO Secretariat must take to achieve these goals.
To support countries in achieving global hepatitis targets under the 2030 Agenda for Sustainable Development, WHO is working in the following areas:
- raising awareness, facilitating partnerships and mobilizing resources;
- developing evidence-based policy and collecting data to inform action;
- improving health equity in the hepatitis response;
- prevention of transmission of infection;
- expanding coverage of screening, care and treatment services.
Since 2011, WHO has worked with countries, civil society and partners to organize annual events to mark World Hepatitis Day (one of the nine major annual health campaigns) to raise awareness and understanding of the virus. hepatitis A. This day is held on July 28 to honor the birthday of Nobel Prize-winning scientist Dr. Baruch Blumberg, who discovered the hepatitis B virus and developed a diagnostic test and vaccine against the virus.
In 2021, WHO is celebrating World Hepatitis Day under the theme “The fight against hepatitis can't wait” to highlight the urgency of eliminating hepatitis in order to achieve the goals by 2030. Key messages relate to the latest estimates of the burden of viral hepatitis and mortality from viral hepatitis at global and regional levels, and the need to ensure that hepatitis is certified as a public health threat by 2030. In addition, recommendations and guidance on self-testing for hepatitis C have recently been published and are expected to support increased testing coverage among key and vulnerable people. population groups, as well as groups with a high burden of HCV infection.
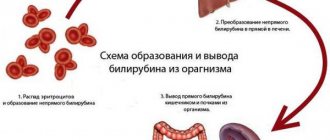
How does hepatitis C occur?
There are two forms of hepatitis C virus: acute and chronic. The acute form is most often asymptomatic and is diagnosed only by chance when markers of acute hepatitis C are detected in the blood - anti-HCV-IgM, which remains in the blood for no more than 6 months after infection with the virus.
After suffering from acute viral hepatitis C, there are three possible scenarios:
- About 20% of patients experience complete recovery;
- In 20% of patients, inactive chronic viral hepatitis C develops with the absence of laboratory markers of the inflammatory process in the liver;
- The remaining 60% have chronic hepatitis with clinical and laboratory manifestations of liver damage.
The transition of the disease to a chronic form occurs unnoticed. Liver damage increases over the years and the patient develops liver fibrosis with subsequent liver dysfunction. The disease progresses slowly over years. In patients with active hepatitis, the risk of developing cirrhosis within 20 years reaches 20%, of which 5% develop liver cancer.
up
How to legally import unregistered medicine into Russia
Polina Naydenkova lawyer in the field of pharmacology and medicine
There are two legal ways to buy the drug abroad: simple and complex.
Downtime is when the patient himself brings it in his luggage for his treatment. This option cannot be implemented by power of attorney; only legal representatives - parents and guardians - can transport medications. Obviously, not every patient and does not always have such an opportunity - this is a disadvantage of this method. In addition, this method is only suitable for drugs registered in the Russian Federation - you can check the availability of registration through the State Register of Medicines. However, if the drug is a prescription drug, you will need a prescription from your doctor to transport it.
The difficult way is to import it under a special permit issued by the Russian Ministry of Health. Under this permit, medications can be transported not only in person, but also by post.
To obtain such permission, you need to submit a package of documents to the Russian Ministry of Health:
- Protocol of the consultation of a federal specialized medical organization or institution of the Russian Academy of Medical Sciences or an institution of a constituent entity of the Russian Federation in which medical care is provided to a specific patient. This protocol must indicate the need to prescribe the drug for health reasons, and such a need for which the prescription of a drug registered in the Russian Federation is impossible. The protocol is signed by the chief physician of the institution.
- An appeal from the regional health authority about the need to import an unregistered drug, accompanied by a copy of the council’s conclusion.
- An electronic copy of the passport or birth certificate of the patient who is prescribed the drug, certified not by a notary, but certainly by the medical institution providing medical care.
- Application for obtaining permission to import into the territory of the Russian Federation an unregistered medicinal product necessary to provide medical care for the vital indications of a particular patient. The application form was approved by the Russian Ministry of Health.
All this - both on paper and at the same time in the form of an electronic document - is submitted to the address and to the Russian Ministry of Health. The Russian Ministry of Health will review the documents and make a decision within one week. Such an application is not subject to state duty.
Violation of the rules for the import of drugs unregistered in the Russian Federation is subject to administrative and criminal liability. If a medicine is not registered in Russia, and there are no documents confirming the right of a specific individual to receive it, government agencies recognize it as falsified or of poor quality.
According to Art. 6.33 of the Code of Administrative Offenses of the Russian Federation, illegal import of unregistered medicines into the territory of the Russian Federation entails the imposition of an administrative fine on a citizen in the amount of 70,000 to 100,000 RUR.
If the state sees in the actions of an individual a sales goal - this depends on the size of the batch of imported drugs - liability is threatened in the form of imprisonment for a term of 3 to 5 years.
Where to start treatment?
From the examination.
A standard examination provides complete information about the virus, its genotype and viral load, as well as a detailed picture of the condition of the liver. For this purpose, biochemical blood tests are carried out to determine the structural and functional state of the liver cells, ultrasound, and assessment of the degree of fibrosis (using the Fibroscan, FibroMax, Fibrotest methods).
You can get tested:
- from 9:00 to 17:30 on weekdays
- from 9:00 to 15:00 on Saturday
Sign up by phone
+7
Seven days a week from 9:00 to 21:00
Request a call back
An important and necessary part of the examination is to exclude contraindications for prescribing therapy, since the instructions for antiviral drugs prohibit their use for a number of concomitant pathologies.
up
How I thought about treatment
The infectious disease specialist from the local clinic, a woman about 60 years old, was neither happy nor upset when she saw me. The duty officer asked why I had never come in all this time, and wrote me a referral for tests with a week to come for the results.
A week later I was like a bayonet. I found out that everything is bad with me, as it should be given my attitude to health: the viral load - the number of viral particles in the body - is growing, liver enzymes are also elevated, and on ultrasound the liver itself is not well enlarged.
I repented of my sloppiness and promised to improve. The doctor prescribed me medications to maintain liver health - so-called hepatoprotectors, gave me a printout with a special diet and was already ready to say goodbye to me. And then I asked if there was any news in terms of a complete cure for my hepatitis. Her answer surprised me a little:
- There is treatment, but do you need it?
It turns out that since the nineties there has been a treatment regimen for hepatitis C with interferons - special proteins similar to those secreted by the human body in response to the invasion of any viruses. It is believed that this protein is capable of modifying the membrane of uninfected liver cells and thereby protecting them from the penetration of the virus. In general, the scheme works, but there are some nuances:
- The effectiveness, depending on the genotype of the virus, ranges from 45 to 90%.
- The course of treatment lasts from six months to one and a half years.
- Treatment is by daily injections.
- The drugs have a huge list of side effects, some quite serious.
Simply put, it is possible to undergo treatment, but it is long and difficult, with no guarantee of cure and with a high probability of significantly worsening the quality of life as a result. But there is a program, and they will put me on the waiting list if I insist. In the meantime, as a farewell, the doctor gave me a business card with contacts of a local group of patients undergoing interferon treatment.
And here I dived deeply into the topic for the first time. I met and communicated with a girl named Yulia, who at that time had been on the pegylated interferon + ribavirin regimen for almost six months. Pegylated interferon works in the body much longer than usual. Thanks to this, Yulia needed to get an injection once a week, and not daily. But this is every week throughout the year! And you can’t get injections in your hands or give them to yourself. You need to come to the clinic every week. And that's not the worst part.
The most unpleasant thing is the side effects. From the first injections, the temperature rises and a prolonged flu-like state sets in with aching bones. Yulia told me that you get used to it after about a month. Much worse is constant itching of the skin and various digestive disorders. Almost all patients also experience severe depression, which in itself is a serious pathological condition.
And the most painful topic for the female half is hair. During treatment they begin to fall out. I’ll say right away that Yulia endured all these “charms” like a steadfast tin soldier. Working, raising a child. She also constantly encouraged me to start therapy. And thank God, her treatment worked, the virus went away and did not return.
But then I didn’t make up my mind. I read how they give up injections halfway through, when there is no more strength to continue. Or due to lack of response to treatment. I thought about it and didn’t sign up for the program. In addition, with hepatoprotectors, my discomfort gradually subsided, and for several more years I limited myself to symptomatic treatment.
Modern methods of treating viral hepatitis C
The international standard for the treatment of viral hepatitis C is combination therapy with interferon and ribavirin. Doses of drugs and duration of treatment are selected by the doctor individually depending on many factors (see Treatment of viral hepatitis C).
Recently, new drugs with direct antiviral action have appeared, significantly increasing the possibility of complete recovery. Therapy with new drugs is prescribed according to individual regimens.
up
Summary of key recommendations
1. Alcohol screening and counseling to reduce moderate and heavy alcohol consumption
For all persons with HCV infection, it is recommended that alcohol consumption be assessed and, if moderate to high levels of consumption are identified, behavioral interventions to reduce alcohol consumption be offered.
2. Examination of patients to determine the stage of liver fibrosis or cirrhosis
In resource-limited settings, the aminotransferase-to-platelet ratio (APRI) test or FIB-4 test is used instead of other expensive noninvasive tests such as elastography or FibroTest.
Recommendations for the treatment of hepatitis C
3. Examination to determine indications for treatment
All adults and children with chronic HCV infection should be assessed to determine whether antiviral therapy is indicated.
4. Treatment
WHO recommends treatment for all persons aged 12 years and older with known HCV infection, regardless of stage of disease. When treating people over 18 years of age with chronic HCV infection, WHO recommends pangenotypic regimens using DAAs. WHO recommends the following treatment regimens for adolescents with chronic hepatitis C aged 12-17 years and weighing at least 36 kg:
- sofosbuvir/ledipasvir for 12 weeks for genotypes 1, 4, 5 and 6;
- sofosbuvir/ribavirin for 12 weeks for genotype 2;
- sofosbuvir/ribavirin for 24 weeks for genotype 3.
For children under 12 years of age with chronic hepatitis C, WHO recommends:
- delay treatment until the child is 12 years old;
- no longer prescribe courses of interferon-based treatment. New highly effective oral pan-genotypic DAA regimens for children under 12 years of age are expected to become available in late 2021 or early 2021. This will expand access to treatment and make it possible to start treatment for this vulnerable group of patients earlier in the course of the disease.
Key Points
- Chronic viral hepatitis C is accompanied by a number of skin diseases, such as mixed cryoglobulinemia, lichen planus, porphyria cutanea tarda, and necrolytic acral erythema.
- Most often, the pathogenesis of skin diseases associated with chronic viral hepatitis C is associated with the formation of immune complexes.
- In addition, skin lesions can be a side effect of interferon and ribavirin therapy.
- The risk of cutaneous complications of hepatitis C viral therapy has decreased significantly since the advent of new drugs.
Introduction
Chronic viral hepatitis C is a global health problem worldwide.
The disease is associated with fatal liver damage in infected patients. Millions of people around the world suffer from this disease, the highest prevalence is observed in Asia, the Far East, and North Africa. In the United States, about 1.3% of the population is infected, with a significantly higher rate of 3.2% among those born between 1945 and 1965. Chronic viral hepatitis C is a very heterogeneous disease, manifested not only by damage to the liver, but also to other organs, including the kidneys, eyes, musculoskeletal system, skin, and nervous system. The disease is associated with skin diseases such as mixed cryoglobulinemia, lichen planus, porphyria cutanea tarda, and necrolytic acral erythema. The literature describes isolated cases of the association of chronic viral hepatitis C with psoriasis and prurigo nodosa. This article will discuss the main pathogenetic mechanisms, clinical picture and some aspects of the treatment of the above skin diseases.
Mixed cryoglobulinemia
Mixed cryoglobulinemia is one of the most common extrahepatic manifestations of chronic viral hepatitis C. The disease can also be associated with lymphoma and rheumatoid arthritis. The clinical picture is characterized by the appearance of a rash, localized mainly on the skin of the lower extremities. The appearance of the rash is caused by the deposition of immune complexes in the walls of small vessels with the subsequent development of vasculitis. There are three immunohistochemical types of cryoglobulins. Type 1 cryoglobulins consist predominantly of pure monoclonal antibodies. They are associated with multiple myeloma or Waldenström's macroglobulinemia.
Type 2 is characterized by a combination of monoclonal IgM and polyclonal IgG. Most often observed in chronic viral hepatitis C, can occur in chronic viral hepatitis B, Epstein-Barr virus. Type 3 is characterized by the appearance of polyclonal IgG and IgM. This type of cryoglobulinemia can occur with viral hepatitis C and autoimmune diseases, such as SLE. In more than half of the cases of mixed cryoglobulinemia associated with viral hepatitis C, type 2 cryoglobulins are detected, less often - type 3 cryoglobulins. Cryoprecipitates are rich in HCV-RNA.
About 95% of all cases of mixed cryoglobulinemia are associated with chronic viral hepatitis C. According to various sources, the frequency of detection of cryoglobulins in patients with chronic viral hepatitis C ranges from 25 to 50% of cases, while the characteristic clinical picture is observed only in 10-30% of cases . A possible reason for this pronounced difference is the different severity of liver damage, fibrosis and duration of the disease. The risk of cryoglobulinemia is directly proportional to the severity of liver damage.
The three most common symptoms of the disease include palpable purpura, arthralgia, and weakness, called the key symptoms of cryoglobulinemia (1966, Meltzer and Franklin). Precipitation of intravascular immunoglobulin precipitates with a decrease in temperature leads to reversible obstruction of the blood vessels of the kidneys, skin, liver, and peripheral nerves. Clinically, Raynaud's phenomenon and small vessel vasculitis are observed. The most common skin lesion is palpable purpura, and chronic ulcers may also occur. In addition, itching, urticarial elements, and leukocytoclastic vasculitis may occur.
Diagnosis of mixed cryoglobulinemia is based on a characteristic clinical picture, medical history, laboratory detection of hypocomplementemia (especially a decrease in the C4 component), and cryoglobulinemia. Viral hepatitis C is often detected. Rheumatoid factor is found in most patients. As a rule, markers of chronic inflammation are detected - increased ESR, CRP, normocytic anemia. When performing a histological examination, leukocytoclastic vasculitis is determined.
Cryoglobulins and immune complexes are found in the wall of the affected vessels. Thus, mixed cryoglobulinemia is an immunocomplex vasculitis of small vessels with predominant damage to the skin, as well as the kidneys, liver, and nervous system. The disease manifests itself as palpable purpura, weakness and arthralgia. Treatment of chronic viral hepatitis C helps resolve the manifestations of cryoglobulinemia, however, in some cases, rituximab therapy and plasma transfusion are required.
Lichen planus
Lichen planus is an inflammatory disease that can affect the skin, mucous membranes of the mouth and genitals, scalp, nail plates, and sometimes the mucous membrane of the esophagus. In typical cases, the primary morphological elements are itchy polygonal papules with a lilac tint, merging into plaques. The size of the rash varies widely - from miliary to plaques more than 1 cm in diameter.
The disease occurs in less than 1% of the population. People aged 30 to 60 years are more susceptible. No gender or race-specific predisposition was found. Typical localization of rashes is the flexor surfaces of the wrists, forearms, the back of the hands, the skin of the legs, and the genitals. There are several main clinical variants of the disease - ring-shaped, more typical for the genital area, inverse type, localized in large folds, atrophic type, often affecting the lower extremities. New lesions may appear after damage to the skin, for example, at the site of scratching or trauma (positive Koebner phenomenon). As a rule, after the rash resolves, no scars remain, and skin damage is quite limited. However, lesions in the oral cavity tend to be chronic.
The etiology of the disease has not been established. It is assumed that the pathogenesis is based on an autoimmune reaction involving activated CD8+ T lymphocytes sensitized to keratinocyte antigens. In patients with chronic viral hepatitis C, cytokines such as tumor necrosis factor alpha, interferon gamma, interleukins 6 and 8 play a certain role in the pathogenesis of lichen planus. The literature describes a large number of cases of manifestation or worsening of lichen planus during interferon therapy.
Despite the fact that the pathogenetic association between chronic viral hepatitis C and lichen planus has not been definitively established, according to previous studies, there is a statistically significant relationship between these diseases. According to a meta-analysis of 70 studies from 5 continents, it was shown that in a number of patients, infection with chronic viral hepatitis C is accompanied by the development of certain clinical variants of lichen planus.
In patients infected with chronic viral hepatitis C, lichen planus was statistically significantly more common than in the control group. According to another meta-analysis, lichen planus in patients infected with chronic viral hepatitis C was 4.8 times more common than in the control group. However, the incidence of chronic hepatitis C virus infection among patients with LP varies widely: from 4% in Europe to 24% in the Middle East.
There are no recommendations for screening for chronic viral hepatitis C, however, it is advisable to conduct it in ethnic groups with a more pronounced association of these diseases.
Skin manifestations
Diagnosis of LP is based on medical history and clinical picture. It is necessary to examine the patient completely, including the oral mucosa and genitals. If a clinical diagnosis cannot be made, a biopsy is required. Diagnostically significant histological features include characteristic epidermal hyperplasia (“saw-tooth pattern”), thickening of the granular layer, vacuolization of the basal layer, apoptosis of keratinocytes (Sivatte bodies), pronounced infiltration in the area of the dermal-epidermal junction, represented mainly by T-lymphocytes.
Skin lesions in LP are limited in nature and can resolve on their own. The first line of therapy is topical corticosteroids of activity classes 2 and 3. Data on the effect of interferon therapy on the course of LP are contradictory; the literature describes cases of exacerbation of the skin process, as well as resolution of skin rashes during interferon therapy. According to a meta-analysis by Petti and colleagues, treatment of hepatitis C does not always lead to resolution of LP lesions. The standard of care for chronic viral hepatitis C is the use of new interferon-free antiviral therapy regimens. There is insufficient data on the effect of new drugs on the course of LP.
Porphyria cutanea tarda
Porphyrias are a group of congenital or acquired metabolic diseases caused by a decrease in the activity of enzymes involved in the biosynthesis of heme and porphyrins. The most common form of porphyria is porphyria cutanea tarda, which is associated with deficiency of hepatic uroporphyrinogen decarboxylase. Adult patients are affected; the incidence of the disease in the United States is 1:25,000 of the population. Men and women are equally susceptible to the disease, however, taking into account risk factors such as alcohol abuse and hepatitis C infection, the disease is more common in men.
There are several types of the disease. The first type (sporadic) represents about 80% of cases of the disease. In this case, no mutation of the UROD gene is observed, so the dysfunction of the enzyme is limited to the liver. The second type (familial) is associated with autosomal dominant inheritance of a defect in the UROD gene, which is associated with a decrease in enzyme activity in all organs and tissues.
The pathogenesis of porphyria cutanea tarda is based on a deficiency of the enzyme uroporphyrinogen decarboxylase (UROD). UROD is the fifth enzyme of heme biosynthesis and catalyzes the decarboxylation of uroporphyrinogen to form coproporphyrinogen.
Clinical manifestations of porphyria cutanea tarda occur when enzyme activity decreases to 20% of normal. The accumulation of porphyrins in the skin leads to phototoxicity: under the influence of 400 nm light, porphyrins release photons and form reactive oxygen species with subsequent damage to membranes, lipids and proteins. According to a meta-analysis performed by Gisbert and colleagues, about 50% of patients with porphyria cutanea tarda are infected with chronic hepatitis C virus, in southern Europe this figure reaches 70%, and in the United States - about 66%.
The exact mechanisms of the influence of the hepatitis C virus on the functional activity of uroporphyrinogen decarboxylase have not been established. It is believed that hepatitis C virus infection leads to the formation of oxygen free radicals, which in turn reduce hepcidin levels and cause iron overload. These changes indirectly lead to deficiency of the enzyme uroporphyrinogen decarboxylase.
Clinical manifestations of the disease are very diverse and include bullous elements, erosions, milia, hypertrichosis, alopecia, onycholysis and hypopigmentation. Photodamage is more pronounced on exposed areas of the skin, such as the face, neck, back of the hands, forearms, and shins. The lesions may be painful and resolve with scarring accompanied by contractures and calcification, similar to systemic scleroderma. Of the laboratory indicators, noteworthy is the often observed increase in the level of transaminases and other markers of liver damage (associated with the presence of viral hepatitis C, alcohol abuse). Patients with porphyria cutanea tarda have an increased risk of developing cirrhosis and hepatocellular carcinoma.
As a rule, a characteristic clinical picture allows one to suspect a diagnosis of “porphyria cutanea tarda”; it is confirmed in the laboratory by detecting an increased level of porphyrins. It is necessary to examine porphyrins in plasma, serum, and urine. In patients with symptomatic porphyria cutanea tarda, urine uroporphyrin and hepatocarboxyl porphyrin are significantly elevated. Another important diagnostic sign is the fluorescence of plasma porphyrins in rays with a wavelength of 620 nm. It is important to conduct screening to detect chronic viral hepatitis C.
Standard treatments include phlebotomy and/or low-dose hydroxychloroquine. In addition, it is important to minimize risk factors - alcohol abuse, taking estrogens, iron supplements, smoking. It is also important to limit exposure to sunlight by photoprotection and wearing closed clothing.
Treatment of chronic viral hepatitis C with interferon preparations did not have a positive effect on the course of porphyria cutanea tarda; moreover, patients with concomitant porphyria cutanea tarda respond worse to interferon therapy. A more acceptable response is observed with combination therapy using interferon and reducing iron load. Despite the limited literature data, the most optimal drugs for the treatment of porphyria cutanea tarda associated with chronic viral hepatitis C are direct antiviral drugs. Thus, porphyria cutanea tarda is a disease caused by a malfunction of the UROD enzyme, manifested by the appearance of bullous lesions on the skin.
Quite often there is an association of porphyria cutanea tarda with chronic viral hepatitis C, therefore, if porphyria cutanea tarda is suspected, it is necessary to exclude infection with the hepatitis C virus. The results of therapy with interferon group drugs are very contradictory. More optimal results can be achieved with the use of new antiviral agents.
Other skin manifestations
Psoriasis
Psoriasis is a common chronic immune-mediated inflammatory skin disease, the main manifestation of which is the appearance of erythematous papules with clear borders, covered with silvery-white scaling. Psoriasis is associated with systemic inflammation, increased production of proinflammatory cytokines - tumor necrosis factor alpha, interferon gamma, interleukin 17. According to a number of epidemiological studies, there is an association between hepatitis C and psoriasis. Two studies from Japan found an increased incidence of serological markers of chronic hepatitis C virus in patients with psoriasis compared with the general population.
According to the literature (Chun and colleagues), increased levels of proinflammatory cytokines during hepatitis C virus infection contribute to the development of psoriasis in genetically predisposed individuals. However, according to an epidemiological study conducted by Kanada and colleagues, no association was found between psoriasis and chronic hepatitis C virus infection. Thus, more research is required to establish the relationship between psoriasis and chronic hepatitis C virus infection.
Necrolytic acral erythema
Necrolytic acral erythema is a rare psoriasis-like dermatological disease characterized by the appearance of itchy erythematous or hypopigmented plaques with clear boundaries and superficial peeling, localized mainly on the skin of the lower extremities. The histological picture resembles psoriasis - papillomatosis, psoriasiform changes, and keratinocyte necrosis are detected. According to various studies, the disease in almost 100% of cases is associated with chronic viral hepatitis C (or zinc deficiency). The first line of therapy is topical and systemic corticosteroids. In addition, modern antiviral therapy leads to positive dynamics in the skin process.
Itching
Itching is a common manifestation of chronic viral hepatitis. Itching is often caused by concomitant cholestasis. More than 15% of patients with chronic viral hepatitis C suffer from severe itching. As a rule, itching is generalized, occurs on unchanged skin, and is accompanied by secondary clinical manifestations - excoriation and lichenification.
There is a frequent association of chronic viral hepatitis C with prurigo nodosa and lichen simplex chronicus. In some cases, itching is associated with the use of antiviral drugs - therapy with interferon and ribavirin is accompanied by severe itching in more than 10% of patients. However, first-generation protease inhibitors cause itching much more often - in more than 40% of patients. The itching in this case was accompanied by a polymorphic rash, which in some patients resembled Stevens-Johnson syndrome. New treatment regimens that do not include interferon and ribavirin are usually not accompanied by itching and rash.
Conclusion
Chronic viral hepatitis C is a systemic disease associated with various extrahepatic manifestations, including skin lesions. Despite the fact that the literature describes a significant number of skin diseases observed in patients with chronic viral hepatitis C, the relationship has been proven only with mixed cryoglobulinemia, lichen planus, porphyria cutanea tarda, and necrolytic acral erythema. The emergence of new drugs for the treatment of chronic viral hepatitis C has led to a decrease in complications of antiviral therapy, including dermatological ones. Additional studies are required to assess the effectiveness of new antiviral drugs on the course of skin diseases associated with chronic viral hepatitis C.
Hepatitis prevention
As with any other disease, hepatitis also requires preventive measures:
- maintain personal hygiene (hepatitis is transmitted through biological material);
- timely implementation of sanitary and anti-epidemic measures;
- regular vaccination.
You need to be more attentive to your health:
- exclude alcohol;
- medications that negatively affect the liver;
- toxic substances; adherence to daily routine and diet;
- undergo regular examinations, monitor blood readings and follow the doctor’s recommendations.