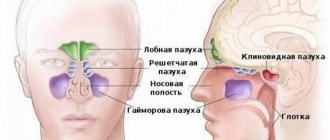
general characteristics
In the lumen of the tracheobronchial tree of a healthy person, up to 100 ml of transparent mucus collects during the day, which has bactericidal properties, participates in metabolism, elimination of infectious agents and small foreign particles from the respiratory tract.
The formation of secretion does not cause coughing or discomfort and goes unnoticed. Sputum is produced in excess quantities in diseases of the nose, its paranasal sinuses, respiratory and digestive organs. The volume of discharge varies depending on the pathological process, in some diseases it reaches 4,000 ml per day. Sputum may come out easily or be difficult to cough up. Mucus often contains blood impurities and foreign inclusions (dust, metal particles, microliths). In most cases, bronchial secretions are odorless; the color is influenced by the nature of the underlying disease.
Mucolytic agents in the treatment of chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD), regardless of severity, is a chronic inflammatory process affecting predominantly the distal airways. An important role in the development and further progression of bronchial inflammation is played by exposure to tobacco smoke, environmental pollutants, and infectious agents [13, 14]. One of the main clinical manifestations of inflammation of the respiratory tract mucosa is cough with sputum production.
The process of formation of bronchial secretion and its movement in the proximal direction is one of the protective functions of breathing. The layer of bronchial mucus humidifies the inhaled air, normalizes its temperature, precipitates and evacuates dust, and fixes microbes and their toxins. Bronchial secretion not only mechanically protects the epithelium from microorganisms, but also has a bacteriostatic effect. The normal daily volume of bronchial secretion ranges from 10-15 to 100-150 ml, or an average of 0.1-0.75 ml per 1 kg of body weight. A healthy person usually does not feel an excess of bronchial secretion; in addition, it does not cause a cough reflex, since there is a physiological mechanism for removing mucus from the tracheobronchial tree - mucociliary clearance (transport) (MCC). It is ensured through the coordinated activity of ciliated cells, which are located in the structure of the multirow prismatic ciliated epithelium. On their free surface there are about 200 ciliated cilia, making 15-16 vibrations per second and moving the mucus layer at a speed of 4-10 mm per minute. Contact of mucus with the cell surface does not exceed 0.1 s, which limits the time of contact of bacteria with the cells of the bronchial mucosa, the possibility of their adhesion and intracellular invasion. Through the MCC, bronchial secretions are transported to the pharynx and then swallowed. MCC is the most important protective mechanism of the respiratory system, ensuring cleansing of the lungs from various inhaled substances, metabolic products, etc. [1, 5, 10].
Bronchial secretions are produced by several types of cells. Goblet cells—unicellular glands of the mesocrine type—secrete a mucous secretion. Their maximum number is observed in the extrathoracic part of the trachea; as the diameter of the bronchi decreases, their number progressively decreases, and in bronchioles less than 1 mm they are completely absent. In a healthy person, the ratio of ciliated to goblet cells is 10:1. Clara secretory cells synthesize phospholipids and bronchial surfactant. They are most numerous in the small bronchi and bronchioles. It is believed that it is they who turn into goblet cells during the development of inflammation in the tracheobronchial tree. Alveolar type II pneumocytes synthesize alveolar surfactant, which, in addition to maintaining the surface tension of the alveoli and improving their distensibility, takes part in the transport of foreign particles from the alveoli to the airways, where, in fact, mucociliary transport begins. Submucosal bronchial glands, related to the glands of the tubular-acinous type, secrete a mucous-serous secretion. Plasma cells, located over the entire surface of the mucous membrane of the tracheobronchial tree, produce immunoglobulins (in the proximal sections they produce mainly IgA, and in the distal sections - IgG). IgA prevents the fixation of bacterial toxins to the mucous membrane and their penetration into the deeper layers of the bronchial wall. At the same time, bacteria are agglutinated and eliminated in sputum [10].
Normally, bronchial mucus consists of 89-95% water, which contains ions Na+, Cl-, Ca+, etc. This liquid part of sputum is necessary for normal mucociliary transport. The consistency of the sputum depends on the water content in the gel. The “dense” part of the bronchial secretion consists of insoluble macromolecular compounds: high and low molecular weight glycoproteins (mucins) (2-3%), represented by two subtypes: neutral (fucomycins) and acidic (sialomucins and sulfamucins), the ratio of which determines the viscous nature of the secretion ; complex plasma proteins - albumins, globulins, plasma glycoproteins (the molecules of which are interconnected by disulfide and hydrogen bonds); immunoglobulins classes A, G, E (2-3%); antiproteolytic enzymes - (1-antichymotrypsin, (1-antitrypsin (1-2%); lipids - mainly phospholipids of bronchial and alveolar surfactant and a small amount of glycerides, cholesterols and free fatty acids (0.3-0.5%). Bronchial secretion characterized by certain physicochemical properties, and primarily by rheological characteristics such as viscosity and elasticity, on which its ability to flow depends [1, 9, 10].
According to the physicochemical structure, bronchial secretion is a multicomponent colloidal solution, which consists of two phases: sol and gel. Sol - a liquid, soluble phase, is a deep layer 2-4 microns thick, which is adjacent directly to the mucous membrane, cilia float and contract in it, the energy of which is transferred to it without delay. The sol contains electrolytes, serum components, locally secreted proteins, biologically active substances, enzymes and their inhibitors. Sol is produced in the respiratory zone (alveoli and respiratory bronchioles), where it participates in air purification, as it has moderate adhesive properties. As the secretion moves further, the contents of goblet cells and seromucoid glands are added to it, forming a gel. The gel, an insoluble, viscoelastic phase, is the upper, outer layer of bronchial secretion, 2 µm thick, located above the cilia. The gel consists of glycoproteins that form a fibrillar structure, which is a wide cellular network, the elements of which contain hydrogen bonds. The gel is able to move only after the minimum shear stress (yield stress) has increased, that is, when the interconnected rigid chains are broken. The ratio of the two phases of gel and sol is determined by the activity of the serous and mucous glands. The predominant activity of the serous submucosal glands leads to the formation of a large amount of secretion with a low content of glycoproteins - bronchorrhea. In contrast, hyperplasia of mucus-forming cells with an increase in their functional activity, observed in chronic bronchitis, bronchial asthma, etc., is characterized by an increase in the content of glycoproteins, the gel fraction and, accordingly, an increase in the viscosity of bronchial secretions [1, 10].
The adhesive properties of the secretion, due to its connection with the dense surface of the bronchi, are also of certain importance. Adhesion reflects the ability for parts of the bronchial secretion to be torn off by air flow during a cough and depends on the condition of the surface of the bronchial mucosa, their ability to be wetted by mucus and the characteristics of the secretion itself.
Thus, the bronchial secretion is a complex complex consisting of the secretion of the bronchial glands and goblet cells, surface epithelium, metabolic products of motile cells, alveolar surfactant, and tissue transudate. In its pure form, bronchial secretions can only be obtained by bronchoscopy. In clinical practice, the concept of sputum is more often used; the latter consists of bronchial secretions and saliva (see figure) [1].
In response to exposure to damaging infectious and non-infectious agents, the first reaction of the mucous membrane of the tracheobronchial tree is the development of an inflammatory reaction with hypersecretion of mucus and restructuring of the mucous membrane, especially the epithelium. Up to a certain point, hyperproduction of mucus is protective in nature, but subsequently not only the quantity, but also the quality of bronchial secretion changes, which disrupts the drainage function of the bronchi and affects bronchial patency. The secretion-forming elements of the inflamed mucosa begin to produce viscous mucus, as its chemical composition changes - the content of glycoproteins increases, a shift occurs towards the predominance of neutral mucins and a decrease in acidic ones, which leads to an increase in the gel fraction, its predominance over the sol and, accordingly, to an increase in viscosity. elastic properties of bronchial secretions. This is also facilitated by a significant increase in the number and area of distribution of goblet cells up to the terminal bronchioles. The adhesiveness of sputum also increases significantly, which reflects a violation of the integrity of the bronchial mucosa and the physicochemical properties of sputum. In parallel with the increase in the volume and viscosity of sputum, a decrease in its elasticity is observed due to an increase in the activity of proteolytic enzymes of bacterial origin and neutrophil elastase of leukocytes. A change in the viscoelastic properties of bronchial secretions is accompanied by significant qualitative changes in its composition: a decrease in the content of secretory IgA, interferon, lactoferrin, lysozyme, which are the main components of local immunity and have antiviral and antimicrobial activity [6, 9, 10].
The deterioration of the rheological properties of bronchial secretions also leads to impaired mobility of the cilia of the ciliated epithelium, which blocks their cleansing function. As viscosity increases, the speed of sputum movement slows down or stops altogether. Thick and viscous bronchial secretions with reduced bactericidal properties are a good breeding ground for various microorganisms (viruses, bacteria, fungi). An increase in viscosity and a slowdown in the rate of movement of bronchial secretions promotes fixation, colonization and deeper penetration of microorganisms into the thickness of the bronchial mucosa, which leads to aggravation of the inflammatory process, an increase in bronchial obstruction, and the formation of oxidative stress. All this contributes to the development of centrilobular emphysema, respiratory failure and cor pulmonale. The formation of emphysema leads to a gradual loss of the reversible component of bronchial obstruction and an increase in its irreversible component. It is in the early stages of the disease that reversible obstruction predominates, which consists of three components: spasm of smooth muscles, inflammatory edema of the bronchial mucosa, hypersecretion and discrimination of bronchial secretions in combination with a violation of the MCC [6, 12].
Thus, when treating patients with COPD, it is necessary to use drugs that improve or facilitate the separation of pathologically altered bronchial secretions, prevent mucostasis and improve the MCB. With the facilitation of secretion, one of the important factors of reversible bronchial obstruction is eliminated, and the likelihood of microbial colonization of the respiratory tract is reduced. This is achieved largely through the use of mucolytic (mucoregulatory) drugs [10]. However, it should be remembered that according to the mechanism of action, mucolytics are not means of influencing the main link of COPD - the inflammatory reaction. They are used during symptomatic therapy, as they affect the symptoms of the disease [6].
The most common are three groups of mucolytic drugs: ambroxol, acetylcysteine, carbocisteine and their derivatives.
Ambroxol (lasolvan, ambrosan, ambrobene, ambrohexal, mucosolvan, chalixol) (see table) is an active metabolite of bromhexine (N-desmethyl metabolite). Derivatives of ambroxol chloride and hydrochloride are successfully used in wide therapeutic practice. Ambroxol has a secretolytic and secretokinetic effect, restores MCC, increases the penetration of antibiotics into the lung tissue. It stimulates the formation of tracheobronchial secretion of low viscosity. The ability of ambroxol to restore MCC by stimulating the motor activity of the cilia of the ciliated epithelium is also important. A distinctive feature of ambroxol and its derivatives is the ability to increase the production of surfactant by increasing its synthesis, secretion and inhibiting its breakdown. As one of the components of the local lung defense system, surfactant prevents the penetration of pathogenic microorganisms into epithelial cells. The surfactant also enhances the activity of the cilia of the ciliated epithelium, which, in combination with improving the rheological properties of bronchial secretions, leads to a pronounced expectorant effect.
In recent years, studies have appeared whose authors point to the anti-inflammatory and antioxidant properties of ambroxol, which can be explained by its effect on the release of oxygen radicals and interference with the metabolism of arachidonic acid at the site of inflammation [22]. However, these data need further clarification [6].
Ambroxol does not have a teratogenic effect, so it can be used in pregnant women. The daily dose of the drug when taken orally ranges from 60 to 120 mg. Typically, adults and children over 12 years of age are prescribed 30 mg tablets or 4 ml of solution 3 times a day in the first three days, and then twice a day. The course of treatment with average therapeutic doses is usually 7–10 days. In severe chronic renal failure, it is necessary to reduce the dose or increase the intervals between doses. Side effects are rare and manifest themselves in the form of nausea, abdominal pain, allergic reactions, dry mouth and nasopharynx. The drug is not used in conjunction with antitussives, as this contributes to the accumulation of bronchial secretions in the respiratory tract.
Bromhexine (bisolvone, bronchosan, phlegamine, fulpen) is a synthetic derivative of the alkaloid vasicine, which has been used in the East as an expectorant since ancient times. When taken orally, bromhexine is converted into an active metabolite, ambroxol, and its effect is similar to that of ambroxol, although less pronounced. Bromhexine is administered orally in a daily dose of 32-48 mg, divided into 2-3 doses. Unlike ambroxol, in severe liver failure the clearance of bromhexine decreases, so adjustment of the dose and dosage regimen is necessary. The drug may accumulate when used repeatedly. It is not recommended for pregnant women and nursing mothers [1].
Acetylcysteine (mucomist, mucobene, ACC, fluomycil) (see table) is an N-derivative of the natural amino acid L-cysteine. N-acetylcysteine derivatives are active mucolytic drugs. These drugs are characterized by a direct effect on the molecular structure of mucus. The acetylcysteine molecule contains sulfhydryl groups, which break the disulfide bonds of acidic mucopolysaccharides of sputum, depolymerization of macromolecules occurs and sputum becomes less viscous and adhesive, and is easier to separate when coughing. The liquefaction of sputum is also caused by stimulation of mucosal cells, the secretion of which has the ability to lyse fibrin and blood clots. The drug is effective for both purulent and mucous sputum. Data on the effect of acetylcysteine on mucociliary transport are contradictory [1, 2].
An important property of acetylcysteine is its ability to stimulate glutathione synthesis by enhancing the activity of glutathione-S-transferase, which takes part in detoxification processes [16]. A significant advantage of acetylsteine is its antioxidant activity, which is realized in various ways. The drug increases the intracellular concentration of glutathione, which performs a protective function in the respiratory system, preventing the action of oxidizing agents. Acetylcysteine also has a direct anti-enzyme effect on free radicals. In addition, it reduces the production of free radicals by alveolar macrophages and enhances the phagocytic activity of monocytes, polymorphonuclear macrophages [15, 17, 20]. Acetylcysteine has certain protective properties directed against reactive oxygen metabolites, free radicals responsible for the development of inflammation in the airways, which is especially important for heavy smokers and elderly patients in whom oxidative processes are activated and the antioxidant activity of blood serum decreases [2, 6, 10 , 18].
Acetylcysteine is prescribed orally at a dose of 200 mg 3 times a day (maximum daily dose 1200 mg) for 1-2 weeks, the duration of its use can be increased to 6 months. Acetylcysteine can also be used in the form of intrabronchial instillations of 1 ml of a 10% solution and bronchial lavage during therapeutic bronchoscopy. There is evidence that long-term use of acetylcysteine in COPD leads to a decrease in the frequency, severity and duration of exacerbations [19, 21]. However, high doses and long-term intake of acetylcysteine can reduce the production of IgA and lysozyme, as well as suppress the activity of ciliated cells, which leads to disruption of the MCB. Undesirable in some cases, especially with intratracheal administration of the drug, is excessive liquefaction of sputum, which can cause the syndrome of “flooding” of the lungs and in this case requires the use of suction [10]. Among the side effects, in some cases, disturbances in the functioning of the digestive tract (nausea, vomiting, heartburn, diarrhea) are observed, and hypersensitivity in the form of urticaria and bronchospasm occurs occasionally.
Among acetylcysteine preparations, the greatest activity is observed in fluimucil. This drug has the least pronounced side effects, since it almost does not irritate the gastrointestinal tract. An important advantage of fluimucil is the possibility of using its solution through a nebulizer in the complex therapy of patients with COPD, taking into account not only the mucolytic properties of the drug, but also its antioxidant activity [6]. It also protects a1-antitrypsin from the inactivating effect of HOCl, a powerful oxidizing agent produced by the myeloperoxidase enzyme of active phagocytes, and also reduces the adhesion of bacteria to the epithelial cells of the bronchial mucosa.
Carbocisteine (broncator, mucodin, mucopront, fluditec, fluifort) (see table) has both a mucolytic and mucoregulatory effect. As a mucolytic, it reduces the viscosity and stringiness of bronchial secretions, ensuring its expectoration, and as a mucoregulator, it increases the synthesis of sialomucins. The mechanism of action of carbocisteine is associated with the activation of sialic transferase, an enzyme of goblet cells of the bronchial mucosa, which form the composition of bronchial secretions. At the same time, under the influence of carbocysteine, regeneration of the mucous membrane occurs, restoration of its structure, reduction (normalization) of the number of goblet cells, especially in the terminal bronchi, and, consequently, a decrease in the amount of mucus produced. In addition, the secretion of immunologically active IgA (specific protection) and the number of sulfhydryl groups (nonspecific protection) are restored, and the MCC is improved, since the activity of ciliated cells is potentiated. In addition to the direct effect on the mucinogenic cell, other effects were identified: antichemotactic, antioxidant and ion-regulatory [9]. The effect of carbocysteine extends to all parts of the respiratory tract involved in the pathological process - upper and lower, as well as the paranasal sinuses, middle and inner ear.
Carbocisteine preparations are available only for oral administration (in the form of capsules, granules and syrups). Average daily doses for adults: one capsule or measuring spoon 3 times a day. Typically, the duration of treatment ranges from 8–10 days to 3 weeks. Long-term use of the drug is possible for 6 months. For long-term use, the drug is used 2 times a day. At the beginning of treatment, after 3-5 days, the volume of sputum increases, and later (by the 9th day) decreases [10].
Side effects include nausea, bowel movements, and abdominal pain. When prescribing carbocysteine preparations, certain precautions should be observed: it is not advisable to simultaneously use drugs that suppress bronchial secretory function and cough suppressants. Carbocysteine preparations should not be prescribed to patients with diabetes mellitus, since one tablespoon of syrup contains 6 g of sucrose. It is not recommended to use carbocisteine for pregnant and nursing mothers [6].
Fluifort is a carbocisteine lysine salt. Lysine increases the water solubility of carbocisteine, ensuring rapid and complete absorption; neutralizes the acidity of carbocisteine, reducing the risk of side effects from the gastrointestinal tract. Fluifort continues to act for 8 days after stopping the drug.
The use of proteolytic enzymes as mucolytics is currently not recommended due to possible damage to the pulmonary matrix and a high risk of serious side effects such as hemoptysis, allergic reactions and bronchospasm [11].
It is possible to use phytotherapeutic agents [1, 8]. The mechanism of action of medicinal herbs is multifaceted, which is associated with the action of various alkaloids and saponins contained in them. The advantage of herbal preparations is that biologically active substances isolated from medicinal plants are more naturally included in the body's metabolic processes (than synthetic ones). They are noted to be better tolerable and less likely to develop side effects and complications. The current level of development of the pharmaceutical industry makes it possible to produce high-quality combined herbal preparations containing optimally selected dosages of active ingredients, for example, Suprima-Broncho cough syrup.
Recently, a new drug, fenspiride (erespal), has been used to treat bronchopulmonary diseases accompanied by broncho-obstructive syndrome. It does not have directly mucolytic and expectorant properties, but due to the anti-inflammatory effect of this drug it can indirectly be classified as a mucoregulator. Erespal affects the main links of the inflammatory process in the respiratory tract and has tropism for the respiratory system. It reduces swelling of the bronchial mucosa and hypersecretion, significantly increases the rate of MCC and counteracts bronchoconstriction, which leads to improved sputum separation, reduced shortness of breath and cough [3, 7].
In accordance with the Federal program (1999) [11], which presents recommendations for the treatment of COPD, mucolytic drugs are prescribed during the period of remission in the presence of symptoms of mucostasis in patients with COPD of any severity, as well as during exacerbation of the disease.
Usually, average therapeutic doses of drugs are prescribed, available in the form of tablets, syrups, drops, “effervescent” tablets, for a period of 9-14 days, and in some cases longer. The duration of taking mucolytic drugs depends on the achievement of the clinical effect, which is assessed based on the improvement in the patient’s well-being and quality of life; changes in symptoms (reduction or disappearance of shortness of breath, reduction and relief of cough, change in the nature of sputum); improvement of external respiration function indicators. It should, however, be taken into account that in a number of patients with chronic bronchitis, after the first day of treatment, the adhesion and viscosity of sputum may increase significantly as a result of the separation of sputum that has accumulated in the bronchi and contains a large amount of cellular detritus, inflammatory elements, proteins, etc. In subsequent days days, with the correct choice of drug, the rheological properties of sputum improve approximately on the 4th day of use of expectorant drugs, its quantity significantly increases, viscosity and adhesion decrease, and on the 6-8th day of treatment the clinical effect stabilizes [10].
When treating patients with COPD, good results can be achieved by prescribing a combination of mucolytic drugs and bronchodilators. The presence of viscous sputum prevents the access of inhaled drugs to the bronchial mucosa. Therefore, ensuring expectoration and freeing the bronchial mucosa from mucus helps to enhance the effectiveness of the drugs and reduce their dose. On the other hand, bronchodilator therapy potentiates the effect of mucolytics and enhances their activity. β2-agonists (formoterol, salbutomol, terbutaline) and theophylline are known to potentiate mucociliary clearance; M-anticholinergics (ipratropium bromide) and theophylline, reducing inflammation and swelling of the mucous membrane, facilitate sputum discharge [5, 10].
In case of severe COPD in remission, in case of exacerbation of moderate and severe disease, administration of drugs through a nebulizer is indicated. For this, special solutions of ambroxol (lazolvan) and acetylcysteine (fluimucil) are used.
Lazolvan is available as a solution for inhalation, 100 ml in a bottle (1 ml of solution contains 7.5 mg of ambroxol hydrochloride). Prescribe 2-3 ml of solution for inhalation, 1-2 times a day. Before use, the drug is mixed with saline in a 1:1 ratio. Lazolvan is contraindicated if there is a history of hypersensitivity to ambroxol.
Fluimucil (acetylcysteine) is a solution for inhalation in ampoules of 3 ml (100 mg of N-acetylcysteine in 1 ml). Prescribe 6 ml of a 5% solution once a day. If necessary, the dose of the drug can be increased. Saline solution is used as a solvent. It can be divided into 2-3 inhalation doses. Fluimucil is contraindicated in case of hypersensitivity to acetylcysteine. It is prescribed with caution to patients with bronchial asthma. If bronchospasm occurs, the drug should be discontinued.
To avoid a cough reflex caused by a deep breath during inhalation, the patient should breathe calmly. It is recommended to warm the inhaled solution to body temperature. Patients with bronchial asthma are recommended to take inhalations after using bronchodilators. Considering that bronchodilator therapy in the treatment of COPD is basic, as well as the fact that it potentiates the effect of mucolytics, it is possible to use lazolvan together with bronchodilators in the same nebulizer chamber.
With exacerbation of COPD, the importance of infectious factors increases, which requires the prescription of antibacterial agents. However, during antibacterial therapy, the viscosity of sputum increases markedly due to the release of DNA due to the lysis of microbial bodies and leukocytes. In addition, thick viscous sputum is a significant obstacle to the penetration of antibiotics into the bronchial mucosa and bronchial secretions. In this regard, it is necessary to carry out measures aimed at improving the rheological properties of sputum and promoting its better discharge. One of these methods is the prescription of mucolytics in combination with antibiotics. Their combined use reduces the period of unproductive debilitating cough by half [10].
When prescribing mucolytics and antibiotics simultaneously, information about their compatibility should be taken into account. Ambroxol, bromhexine and carbocisteine, when used in combination with antibiotics, enhance the penetration of the latter into bronchial secretions and the bronchial mucosa, increasing their effectiveness. This is especially true for amoxicillin, cefuroxime, erythromycin, doxycycline, rifampicin and sulfonamide drugs. Thus, carbocisteine enhances the effect of antibiotics at the bronchial level by 20%. When prescribing acetylcysteine orally, antibiotics (penicillins, cephalosporins, tetracyclines) should be taken no earlier than 2 hours after taking it. Acetylcysteine preparations for inhalation or instillation should not be mixed with antibiotics, as this results in their mutual inactivation [10]. The exception is fluimucil, for which a special form has been created: fluimucil + antibiotic IT (thiamphenicol glycinate acetylcysteinate). Fluimucil is available for inhalation, parenteral, endobronchial and local use. Thiamphenicol glycinate acetylcysteinate (this is a complex compound that combines the antibiotic thiamphenicol and the mucolytic fluimucil. Thiamphenicol has a wide spectrum of antibacterial action and is effective against bacteria that most often cause respiratory tract infections. Fluimucil effectively thins sputum and facilitates the penetration of thiamphenicol into the area of inflammation, inhibits the adhesion of bacteria on the epithelium of the mucous membrane of the respiratory tract [6].
Despite the positive effects that are observed with the use of mucolytic, mucoregulatory agents, data on their use in patients with COPD are very contradictory. Due to the mucolytic properties of these drugs, their ability to reduce adhesion and activate mucociliary clearance, they have proven themselves in the treatment of COPD patients with discrinia and hypersecretion. However, mucoregulators (mucolytics) do not find application points where bronchial obstruction is associated with bronchospasm or irreversible phenomena. Ambiguous data from studies on COPD do not allow the use of these drugs as basic agents in the treatment of patients with this pathology [6]. The GOLD program (2001) [4] notes that although the use of mucolytics (mucokinetics, mucoregulators) in some patients with viscous sputum leads to an improvement in the condition, in general the effectiveness of these drugs is low. From the point of view of evidence-based medicine, reports demonstrating the effectiveness of the use of mucolytics in the treatment of patients with COPD are clearly insufficient (level D). The same program indicates that N-acetylcysteine, as an antioxidant, reduces the frequency of exacerbations of COPD, which may be important in the treatment of patients with frequent exacerbations of the disease (evidence level B). However, before the widespread use of these drugs in medical practice, it is necessary to obtain and carefully evaluate the results of ongoing research [4].
Thus, the prescription of mucolytic drugs is indicated in the complex therapy of patients with COPD, in whom the processes of hypersecretion and discrimination predominate, since these drugs change the rheological properties of bronchial secretions, affect the process of mucus formation, have a normalizing effect on the biochemical composition of mucus, facilitate the separation of sputum, and prevent mucostasis and improve mucociliary clearance. However, mucolytics are not basic therapy for COPD, since they do not directly affect the inflammatory response (the main pathogenetic link of the disease.
For questions about literature, please contact the editor
I. V. Mayev, Doctor of Medical Sciences, Professor G. A. Busarova, Candidate of Medical Sciences
MGMSU, Moscow
Classification
The consistency of sputum can be thick, viscous or thin and watery. When settling, in some cases it is divided into 2 or 3 layers. Based on physical properties (color, smell, transparency, other macroscopic characteristics), the following types of sputum are distinguished:
- Serous.
Isolated in acute left ventricular failure accompanied by pulmonary edema. Characterized by the absence of odor, watery consistency, and an abundance of foam. Serous sputum is usually colorless, sometimes has a pinkish tint. - Mucous.
The appearance of mucous discharge indicates the onset of inflammation of the respiratory tract or the attenuation of the activity of an acute pathological process. Sputum is coughed up in small quantities and is colorless, viscous mucus. - Mucopurulent.
Formed in the acute period of many respiratory diseases. This type of secretion is characterized by increased viscosity and the presence of yellow or green impurities. - Purulent.
Appears with severe inflammation, suppurative processes of the respiratory system. The consistency of the purulent secretion is liquid, the color is green or yellow-green, and sometimes there is a putrid odor.
Perhaps there are shocking photos of medical operations hidden here that show blood and guts.
Are you over 18 years old?
Yes
No
Purulent sputum
Types of sputum
The following is a list of types of sputum and conditions/diseases in which such sputum can occur:
- viscous mucous sputum - bronchospastic processes, bronchial asthma - yellowish sputum with streaks of pus - pneumonia (pneumonia) - rusty sputum - lobar pneumonia - bright red sputum with blood content - necrotic process (infarction) of the lungs - yellow-brown sputum - lung abscess - foamy bloody sputum - pulmonary edema - clear sputum and blood fibers - bronchogenic lung cancer - bloody sputum - lung cancer - sputum with a sweetish unpleasant odor - purulent bronchitis - foul-smelling sputum - gangrene of the lung
Causes of sputum formation
Causes of purulent sputum
The appearance of purulent yellow or yellow-green discharge indicates the presence of a severe acute respiratory infection or an exacerbation of a chronic inflammatory disease of the respiratory tract. Purulent, foul-smelling sputum released with coughing accompanies various destructive processes of the lungs and is observed when pathological contents stagnate in bronchiectasis. The most common causes of purulent secretion are:
- Bronchitis:
acute purulent bronchitis, chronic purulent process in the acute phase. - Bronchiectasis:
bronchitis or mixed variant of COPD, bronchiectasis. - Pulmonary destruction:
lung gangrene, lung abscess. - Neoplasms:
decaying lung cancer.
Causes of thick/sticky sputum
Coughing up viscous mucus or mucopurulent secretion occurs in most diseases of the bronchopulmonary system. Any acute respiratory pathology manifests itself as an unproductive cough with thick, light or yellowish sputum. Chronic diseases are accompanied by the release of viscous secretions during remission. The most common coughing up of thick mucus is observed in the following pathologies:
- Acute respiratory infections:
tracheitis, bronchitis, pneumonia. - Hereditary diseases:
cystic fibrosis, Kartagener syndrome. - Pulmonary mycoses:
candidiasis, aspergillosis, zygomycosis. - Chronic lung diseases:
COPD, bronchial asthma, chronic bronchitis.
Causes of mucous sputum
Some diseases of the respiratory system are manifested by the discharge of thin, watery mucus. Sometimes such sputum is released in single spits, but in a number of diseases there can be a lot of it. Coughing with mucus is often observed in acute airway infections. Mucous sputum, the causes of which are associated with chronic pathology, is coughed up during remission. Excessive production of clear mucus occurs due to the following conditions:
- Manifestation of acute respiratory diseases:
bronchitis, pneumonia. - Remission of chronic respiratory pathology:
COPD, chronic bronchitis. - Attack period of bronchial asthma.
- Infiltrative pulmonary tuberculosis.
- Neoplastic process:
non-small cell cancer, pulmonary adenomatosis. - Digestive diseases:
GERD.
Causes of yellow sputum
Often the sputum secreted by the patient turns yellow due to the activation of bacterial microflora. The appearance of such mucus in the morning may occur due to an admixture of nasal secretions that flow into the trachea during sleep. Sometimes sputum acquires a rich yellow tint due to food colorings and an increased content of eosinophils. The main diseases with the release of yellow sputum:
- Diseases of the nose and paranasal sinuses:
rhinitis, sinusitis. - Acute diseases of the respiratory system:
bronchitis, tracheitis, pneumonia. - Chronic respiratory diseases:
chronic bronchitis, bronchiectasis, COPD. - Eosinophilic infiltrates:
ascariasis, hookworm, drug allergies. - Specific infections:
pulmonary forms of syphilis, tuberculosis.
Causes of mucopurulent sputum
Expectoration of mucus with pus indicates an acute, advanced stage of bronchopulmonary disease. Sometimes mucopurulent sputum is rusty in color and may contain streaks or droplets of blood. Simultaneously with the appearance of pathological impurities, the volume of discharge increases. The main diseases in which there is coughing of mucopurulent contents:
- Acute diseases of the bronchi and lungs:
bronchitis, pneumonia. - Chronic pathology of the respiratory tract:
chronic bronchitis, COPD, the presence of bronchiectasis. - Specific diseases:
tuberculosis of the bronchi, lungs, pulmonary syphilis. - Rare hereditary pathology:
cystic fibrosis. - Malignant neoplasms:
bronchopulmonary carcinoma. - Mycoses of the lungs:
actinomycosis.
Causes of foul-smelling sputum
The strong unpleasant odor of sputum is due to stagnation of the contents of the cavity formations of the lungs, putrefactive processes associated with the activity of anaerobic microflora. When such a cavity is drained into the bronchus, a large amount of foul-smelling semi-liquid secretion is coughed up. Morning sputum sometimes has a strong smell due to impurities in nasopharyngeal secretions. The main pathologies accompanied by this symptom include:
- Suppurative pulmonary diseases:
lung abscess, lung gangrene, pleural empyema. - Neoplastic processes:
disintegration of a cancerous tumor. - Specific infections:
disintegration of syphilitic gumma, drainage of tuberculous cavity. - Bronchiectasis.
- Diseases of the nasopharynx:
ozena.
Causes of green sputum
Green sputum is the result of bacterial infection and stagnation of secretions in the respiratory tube, bronchiectasis, and cavity formations. This discharge usually has a purulent, mucopurulent character, and sometimes has an intense putrefactive odor. Coughing up green contents may indicate a serious bronchopulmonary disease, often observed with pathologies such as:
- Acute diseases of the respiratory system:
bronchitis, pneumonia. - Pulmonary purulent destruction:
gangrene, lung abscess. - Suppurative diseases of the pleura:
pyothorax. - Respiratory tuberculosis:
fibrous-cavernous pulmonary tuberculosis, pleural empyema with tuberculous pleurisy. - Bronchiectasis of any origin.
- Hereditary diseases:
cystic fibrosis.
Diagnostics
Diagnosis of diseases in which sputum is produced is carried out by pulmonologists or therapists. During the survey, the duration of the illness, the presence of occupational hazards, and the connection with smoking are clarified. The examination reveals signs of hypoxia and symptoms of distal hypertrophic osteoarthropathy. The following methods help to finally find out why the patient is coughing up sputum:
- Physical examination.
Local dullness of percussion sound allows one to suspect pneumonia and the presence of pleural effusion. Dry wheezing during auscultation indicates bronchial obstruction, moist and crepitating pathological respiratory sounds are heard during infiltration of the lung tissue. - Visualization methods.
X-ray of the lungs reveals infiltrates of the pulmonary parenchyma, spherical formations, and the presence of lung destruction. CT and MRI of the chest are prescribed to clarify the localization of the pathological process. Signs of pyothorax can be visualized using x-ray or ultrasound of the pleural cavity. - Endoscopic techniques.
Bronchoscopy makes it possible to examine the mucous membrane of the bronchi and trachea. Using this method, you can detect manifestations of bronchitis, bronchiectasis, and areas of bronchostenosis. Under the control of a bronchoscope, a biopsy of suspicious areas of the bronchial wall is performed. Fibrogastroscopy is performed to exclude GERD, rhinoscopy is performed if nasopharyngeal pathology is suspected. - Laboratory research.
Peripheral blood tests reflect inflammatory changes in the body. During a microscopic examination of sputum, its physical qualities are assessed, Kurshman spirals, Dietrich plugs, and other inclusions are identified. The cultural method allows you to detect the causative agent of an infectious disease and determine its sensitivity to antibiotics.
Sometimes, when coughing with sputum, spirometry, body plethysmography, and allergy diagnostics are additionally performed. To exclude syphilis, helminthic infestations, and pulmonary mycoses, serological tests are performed. To diagnose hereditary pathology, molecular genetic methods and special studies are used. To confirm tuberculosis, the Mantoux test, Diaskin and Quantiferon tests can be used.
Bronchoscopy is performed for both diagnostic and therapeutic purposes.
Composition of sputum
Sputum consists of more than 95% water, 5% proteins, carbohydrates and mineral salts.
In patients with respiratory tract diseases, sputum may contain protein, blood, polysaccharide components, bacteria, viruses, and fungal substances. During oncological processes, atypical tumor cells appear in the sputum. In such cases, the ogranoleptic characteristics of the sputum change - it becomes mucous, viscous, may be watery, contains streaks of blood, or becomes bloody in mass. The sputum develops an unpleasant odor and changes color.
Patient sputum analysis and research
Sputum serves as an important diagnostic material for making a diagnosis of respiratory diseases. Based on appearance, the presence or absence of pus or blood in the sputum, as well as microbiological examination data, the doctor can make an assumption about the nature of the pathological process in the patient’s respiratory tract. Based on the appearance, smell, volume and composition of sputum, lung diseases can be divided into infectious, traumatic, oncological, etc. In certain cases, the presence of edema or even necrosis of the lungs can be suspected.
Sputum examination allows the doctor to determine a further diagnostic strategy and select the correct methods of instrumental and laboratory diagnostics. In addition to diagnostics, the characteristics of the sputum of the patient being examined help the doctor take urgent measures to organize antibiotic therapy, hemostatic and decongestant therapy. In some cases, prompt initiation of treatment can save the patient's life.
In all cases when a patient experiences a change in the properties of sputum, the doctor must determine the cause of the condition that led to these changes. After the cause is found, etiotropic treatment is prescribed aimed at eliminating the cause of the disease. In addition, in all cases, nonspecific therapy is administered, with the help of which the volume, viscosity and composition of sputum are normalized.
Treatment
Help before diagnosis
Long-lasting, regularly expectorated sputum is a reason for a mandatory visit to the doctor. If you are coughing up liquid purulent contents with your mouth full, there is hemoptysis or rust-colored sputum, a visit to a medical facility should be urgent. Before making a diagnosis, it is recommended to drink plenty of warm alkaline drinks, expectorant herbal teas and medications, and breathing exercises.
Conservative therapy
There are a large number of pathologies in which sputum is coughed up; treatment depends on the etiology and mechanism of development of the disease. Conservative measures include the use of pharmacological agents and physiotherapeutic procedures, physical therapy. Medicines can be divided into the following groups:
- Etiotropic.
Antibiotics are prescribed for bacterial infections, taking into account the sensitivity of the microflora. When treating tuberculosis, combinations of anti-tuberculosis drugs are used. Antifungal medications are indicated for fungal diseases, anthelmintic medications for helminthic infestations. - Pathogenetic.
Includes expectorants. During treatment, sputum becomes more liquid and is better removed from the bronchi. Preference is given to mucolytics and mucoregulators. The group of pathogenetic agents also includes bronchodilators and corticosteroid hormones used for bronchial obstruction. For malignant neoplasms, antitumor medications are recommended.
Physiotherapeutic treatment is actively used for a number of pulmonary diseases. Patients are prescribed medicinal inhalations and breathing exercises. Sputum is cleared well after percussion massage of the chest. Patients are trained in postural drainage techniques. If necessary, sanitary bronchoscopy is performed.