Pathophysiology of oxidative stress
The basic mechanisms of pathology in any critical condition, including surgical and traumatic aggression, are free radical processes and changes in the properties of cell biomembranes. The main pathological role of free radicals is that they actively interact with molecules that form neuronal and intracellular membranes. The viscosity of the membranes increases, their plasticity and functional state are lost. Along with this, genes responsible for programmed cell death - apoptosis - are activated. There is a direct relationship between the accumulation of lipid peroxidation products and the severity of damage to nerve cells and other tissues.
Since the formation of tissue hypoxia, lipid peroxidation, and mitochondrial dysfunction are recognized as the trigger for the development of a typical pathological process, the use of antihypoxants and antioxidants is pathogenetically justified in surgical and traumatic aggression, inflammation and acute pain.
Restoring blood flow in previously ischemic tissues also poses a certain danger. Reperfusion causes a multiple increase in the partial pressure of oxygen with a further increase in free radical processes. In this case, the endothelium of the capillaries is damaged, the anticoagulant activity of which is transformed into procoagulant activity. Due to increasing adhesion, leukocytes and platelets clog the capillaries. This process is aggravated by an increase in the rigidity of red blood cells, which sharply increases the disruption of oxygenation of tissues, especially the brain. The processes of blood fibrinolysis are inhibited, the area of cerebral infarction expands, and cerebral edema increases.
The main pathological processes initiated by excessive activation of LPO
I. Cellular-tissue level:
- ischemia;
- hypoxia;
- membranopathy:
– disruption of the permeability of the cell membrane and membranes of cell organelles;
– excessive accumulation of free radicals inside the cell;
– release of lysosomal enzymes into the cell;
– accumulation of Ca++ ions inside the cell;
- apaptosis and cell necrosis;
- violation of cellular reception;
- energy and metabolic disorders.
II.
Organs and systems:
- functional disorders;
- organic pathology.
Of course, the body has an endogenous antioxidant system (AOS), but at critical levels of hypoxia and LPO it is untenable and it is necessary to introduce antioxidants from the outside.
Disorders of energy and metabolic processes in cells and tissues
Reasons for initiation (strengthening) gender
- stress;
- ischemia;
- hypoxia;
- tissue reperfusion; (reperfusion syndrome);
- inflammation (aseptic or bacterial);
- insufficient activity of the physiological antioxidant system (relative and absolute).
If patients' own AOS is insufficient, it is necessary to administer antioxidants and antihypoxants as quickly as possible. Only a few of them are used clinically. Reambirin is used quite widely in anesthesiological and surgical practice as an infusion and energy preparation of a polyonic composition, which contains sodium succinate.
Reamberin:
- 1.5% infusion solution:
- Sodium chloride – 6.0 g
- Potassium chloride – 0.3 g
- Magnesium chloride – 0.12 g
- N-(1-deoxy-D-glucitol – 1-
- N-methylammonium sodium succinate – 15 g
- Water for injections – up to 1 l
However, its antioxidant properties for the correction of oxidative stress in surgical practice and in acute pain have not been studied enough. In the anesthetic aspect, the domestic drug Mexidol from the group of synthetic antioxidants and antihypoxants is currently interesting.
Mexidol
- international proprietary name – hydroxymethylethylpyridine succinate
- chemical rational name – 3-hydroxy-6-methyl-2-ethylpyridine succinate
- dosage form: solution for injection 5% in ampoules of 2 ml n 10. One ampoule with 2 ml solution for injection contains 100 mg of the active substance. tablets 125 mg n 30.
- According to the chemical structure, Mexidol is a salt of succinic acid (succinate).
According to available information, Mexidol is an antioxidant, a free radical inhibitor, a membrane protector, reduces the activation of lipid peroxidation, and increases the activity of the physiological antioxidant system as a whole.
Pharmacokinetics of Mexidol
Has high bioavailability. It dissolves well in water. It has high lipophilicity. It quickly passes from the bloodstream into organs and tissues and is quickly eliminated from the body. High lipophilicity and its ability to bind to blood plasma proteins and endoplasmic reticulum membranes suggest the possibility of the formation of its tissue and blood depot.
Due to the presence of a 3-hydroxypyridine derivative in its composition, which is an active carrier, it penetrates into the cell and mitochondria
(Dumaev K.N., 1995).
The maximum concentration at doses of 400-500 mg is 3.5-4.0 mcg/ml. The retention time (MRT) of the drug in the body is 0.7-1.3 hours.
When administered intramuscularly
– the time to reach maximum concentration is 0.45-0.50 hours.
– determined in blood plasma for 4 hours after administration.
the time to reach maximum concentration (Tmax) in blood plasma is 0.46-0.50 hours;
– the half-elimination period (T1/2 et) and the average retention time (MRT) of the drug in the body are 4.7-5.0 hours and 4.9-5.2 hours, respectively.
Intensively metabolized in the liver with the formation of its glucuron-conjugated metabolites. It is excreted by the kidneys mainly in the form of glucurone-conjugated metabolites. On average, over 12 hours, 0.3% of the unchanged drug and 50% in the form of glucuronoconjugate are excreted in the urine.
Toxicology:
- Acute toxicity
– with oral administration LD50 = 4 g/kg body weight, with intravenous administration LD50 = 800 mg/kg body weight. Therapeutic index ND50 /ED50 =6.2, and LD50 /ED50 =16.4 or more - Chronic toxicity
: with long-term use orally and parenterally in experimental animals, no significant changes in organs and tissues were detected.
Contraindications:
- hypersensitivity;
- acute disorders of liver and kidney function
- childhood, pregnancy, breastfeeding (since no clinical trials have been conducted).
Side effects
(extremely rare):
- drowsiness;
- dry mouth.
Side effects (drowsiness, potentiation of other anesthetics, dry mouth can be successfully used in patients as premedication before surgery. After all, we usually deliberately use relanium and atropine for these purposes.
3-hydroxypyridine derivatives
(included in Mexidol)
They play a big role in metabolism.
- Necessary for the normal functioning of the central nervous system.
- They are part of enzymes that carry out decarboxylation and transamination of amino acids.
- Participate in the metabolism of tryptophan, methionine, cysteine, glutamic and other amino acids, histamine.
- Participate in lipid metabolism processes, improving lipid metabolism in atherosclerosis.
Features of the Krebs cycle reaction associated with succinate
Features of the Krebs cycle reaction associated with succinate:
- Oxidation of succinate is a prerequisite for the catalytic effect of the intermediate on the uptake of oxygen by the cell
- To replenish the pool of organic acids, it is enough to introduce one succinate
- The activity of succinate dehydrogenase does not depend on the concentration of NAD and NADH
- The power of an energy production system using nuclear fuel is hundreds of times greater than all other systems
- The phenomenon of rapid oxidation of succinate in the cytoplasm of cells with reduction of dinucleotide
Activation of oxidative stress during surgery and anesthesia is a key link in the development of pathophysiological processes of critical conditions. And the level of oxidative stress is a modern criterion for the adequacy of anesthesia. There is no doubt about the need to include antioxidants and antihypoxants in anesthesia in order to improve the protection of the operated patient from surgical aggression. At the same time, this issue has been practically not studied.
In our clinic, together with the Department of General Surgery (Head Prof. Yu.S. Vinnik), oxidative stress in surgical patients and the possibility of its correction with antioxidants and neuropeptides have been studied for a number of years. In particular, oxidative stress during operations on the biliary tract was studied.
Go to the contents of the book “Pacifying Pain”
Recent decades have been characterized by significant advances in the prevention and treatment of cardiovascular diseases (CVD): arterial hypertension (AH); various forms of coronary heart disease (CHD) – acute coronary syndrome (ACS), myocardial infarction (MI); chronic heart failure (CHF). These achievements are due to the introduction into clinical practice of modern high-tech methods for diagnosing and treating left ventricular failure, which are based on a clear understanding of the mechanisms of development of ischemia and death of cardiomyocytes (structural and functional units of the myocardium), and adaptive changes in central hemodynamics.
IHD is widespread throughout the world, especially in economically developed countries, and occupies a leading place in the structure of disability and mortality from CVD among a socially significant age group of the population. In most European countries, the prevalence of IHD is 20–40 thousand per 1 million population. Mortality from diseases of the circulatory system in the Russian Federation, according to medical statistics for 2010, amounted to 56.5% of total mortality; Of these, more than half account for ischemic heart disease as the cause of death.
IHD as an “independent disease” was identified by the World Health Organization (WHO) only in 1965 due to the increasing frequency of this pathology, its dominant participation in the progression of cardiac pumping disorders in CHF, and was included in the International Statistical Classification of Diseases, Injuries and Causes of Death.
In IHD, there is a discrepancy between the level of oxygen consumption by the myocardium and the volume of its delivery by the coronary bloodstream. Adequate energy supply for the pumping activity of the heart in a wide range of its activity - from rest to the level of maximum load (corresponding to the level of basal metabolism of the whole organism) depends on the state of the coronary reserve. Coronary reserve is the ability of the coronary vascular bed to increase coronary blood flow many times due to dilatation of the coronary vessels adequately to the oxygen needs of the myocardium.
Oxygen is a key component of oxidative phosphorylation in the synthesis of ATP, the “fuel” that ensures the functioning of cardiomyocytes and the pumping activity of the heart in general. Energy metabolism in the myocardium represents interconnected mechanisms of O2 delivery and its utilization by the subcellular structures of the cardiomyocyte - mitochondria [1, 2, 4].
To provide energy for its activity, the heart “utilizes” various biological substrates: carbohydrates (glucose, glycogen, lactate), free fatty acids (FFA), and, to a lesser extent, amino acids (proteins). Regardless of the energy substrate, in the final stage of the breakdown of biological substrates, acetyl coenzyme A is formed, which enters the tricarboxylic acid cycle (Krebs cycle), and with the participation of O2 in the mitochondria, the energy substrate ATP is formed.
Under physiological conditions, 10% of ATP is formed during oxidative phosphorylation in mitochondria due to aerobic glycolysis (the breakdown of glucose to pyruvate). The amount of ATP generated as a result of aerobic glycolysis is not enough to ensure the operation of ion channels of the sarcolemma, in particular for the calcium pump of the sarcoplasmic reticulum (SR), which consumes up to 50% of the synthesized energy to ensure diastolic relaxation. Replenishment of the remaining amount of phosphate energy for the functioning of the cardiomyocyte as a whole, with normal oxygen supply, occurs due to the oxidation of FFA. The metabolism of FAs during oxidative phosphorylation provides up to 80% of ATP synthesis. However, FFA oxidation, compared to glycolysis, is a less efficient source of ATP: “fuel” for the heart pump. When oxidizing FFAs, the production of the same amount of ATP requires approximately 10% more oxygen than during glycolysis [1, 4].
FFAs penetrate into mitochondria through active transport, for which the carnitine palmitine enzyme complex is responsible, then β-oxidation of FFAs occurs in mitochondria. This process is strictly controlled and depends mainly on the intensity of FFA translocation into mitochondria. In the case of moderate ischemia, aerobic oxidation of FFA and glucose decreases and anaerobic glycolysis becomes the main source of ATP. Under these conditions, glycogen reserves are mobilized to support glycolysis.
With the development of varying degrees of ischemia (partial or complete occlusion of the coronary artery), anaerobic glycolysis remains the only source of limited ATP formation. As O2 delivery decreases, the activity of oxidative metabolism decreases, producing a limited amount of ATP. A pronounced imbalance between the oxygen demand during the oxidation of glucose and FFA towards the latter leads to the fact that during ischemia in the mitochondria of cardiomyocytes, ATP synthesis switches to β-oxidation of FA with the accumulation of many under-oxidized active forms of FA acyl-coenzyme-A (Acyl-CoA) and acylcarnitine (AcCar). ), which further exacerbates the uncoupling of oxidative phosphorylation (Fig. 1). Underoxidized active forms of FA, in particular AcCar and Acyl-CoA as metabolites, block the transport of ATP from the site of synthesis in mitochondria to the site of their intracellular consumption, have a destructive effect on the membrane - the sarcolemma, increasing the energy deficit necessary for the life of cardiomyocytes [2, 4, 6, 10].
In parallel, under conditions of severe ischemia (lack of blood flow), lactate and H+ accumulate in cardiomyocytes, i.e., against the background of anaerobic metabolism, protons (H+, Na+) accumulate and “acidification” of the cytoplasm occurs. H+ and Na+ ions are exchanged for other cations (mainly Ca2+), as a result of which the cardiomyocytes are “overloaded” with Ca with the formation of incomplete diastole - myocardial contracture (Fig. 2).
Modern advances in the study of cell function (in particular, endothelium) of various organs indicate the key role of oxidative stress - excessive formation of reactive oxygen species (ROS - O2) in the formation of CVD through lipid peroxidation (LPO) of the cell membrane. The main source of ROS in cells is mitochondria, during the normal functioning of which 98% of the supplied oxygen is used for the oxidation of substrates with the formation of ATP (the main energy substrate of cells) and 2% for the synthesis of ROS, which can increase significantly in various pathological conditions (Fig. 3) .
A decrease or cessation of O2 delivery to the heart muscle can be caused by various mechanisms: from spasm to total blockage of the coronary artery. After restoration of coronary blood flow, damaged mitochondria are not able to completely utilize the “surging” supply of oxygen, part of which is used by other oxidative systems of cells and is accompanied by the formation of an increased amount of ROS. The activity of one of the powerful oxidative enzymes, xanthine oxidase, is at a low level under conditions of aerobic metabolism, but increases sharply under hypoxia, in addition, with the conversion of Fe3+ to Fe2+. The combination of these two factors contributes to the excessive formation of ROS [8]. Excessive formation and release of free radicals (ROS) activate lipid peroxidation (LPO) with damage to cell membranes, which consist of phospholipids, cholesterol and protein inclusions that act as ion channels or receptors.
All of the above is an incentive for clinicians in the treatment and prevention of possible complications in the conditions of ischemic episodes in various regions (heart, central nervous system): the use of drugs with antioxidant and antihypoxic pharmacological orientation, which have pleiotropic effects (cardio-, neurocytoprotection), restoration of aerobic intracellular metabolism. A typical representative of drugs with similar pharmacokinetic and pharmacodynamic properties is Actovegina.
Actovegin is a highly purified hemodialysate from the blood of calves, obtained by ultrafiltration, does not contain endotoxins and antigens and consists of biologically active physiological components with high biological activity: amino acids, oligopeptides, nucleosides, products of carbohydrate and fat metabolism. The pharmacological composition of Actovegin is formed by 2-stage ultrafiltration using filters to isolate molecules of different sizes. The molecular weight of the final filtered product does not exceed 5000 Daltons. The composition of Actovegin was tested using modern analytical techniques, including gas liquid chromatography combined with mass spectrometry. Data from quantitative methods for analyzing possible metabolites showed that Actovegin is a combination of more than 200 bioactive molecules [6, 7, 10].
The atomic emission spectrometry method showed the presence in Actovegin of macro-electrolytes (Mg, Na, Ca, P, K) and microelements (Si, Cu), which are included in the prostatic groups of antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase). The antioxidant effect of Actovegin is due to superoxide dismutase activity [8]. Magnesium, which is included in Actovegin, is a component of cardiopeptide fragments and enzymes and functions as a catalytic center that provides control and launch of enzymobiochemical intracellular processes.
The anti-ischemic effect of Actovegin at the cellular level is carried out due to the transfer of cell energy metabolism towards aerobic glycolysis with inhibition of β-oxidation of fatty acids. Actovegin®, selectively inhibiting 3-ketaocetyl-CoA catalase, slows down the ß-oxidation of fatty acids, while competitively restoring the coupling between glycolysis and oxidative decarboxylation, which overall leads to an increase in the amount of ATP, which underlies the anti-ischemic protection of cardiomyocytes by Actovegin (Fig. 4) .
Experimental studies at the cellular level have shown that Actovegin® supports the energy metabolism of the heart. The cardioprotective effect of Actovegin is due to its ability to maintain the physiological level of creatine phosphate (the main carrier of energy inside the cell) and ATP under conditions of ischemia, stabilize the pH inside the cell (prevents the development of intracellular metabolic acidosis), and reduce damage to the membrane - sarcolemma - by lipid peroxidation caused by free radicals. Normalization of metabolic balance leads to limiting the accumulation of inorganic phosphate, Na and Ca inside the cell while maintaining normal K concentration. At the same time, Actovegin® reduces the level of migration and infiltration of polynuclear neutrophils (inhibition of chemotaxis) in ischemic and reperfused heart tissues, which reduces autoimmune damage to the myocardium without causing influence on central hemodynamics [4, 5, 9].
Oxidative stress causes breakage of 1 strand of DNA, which leads to activation of the nuclear enzyme poly-ADP-ribose polymerase (PARP). Excessive activation of PARP has negative consequences in the form of triggering successive cellular processes that ultimately stop glycolysis and the process of mitochondrial respiration (oxidative phosphorylation - Krebs cycle), which leads to cell death due to energy depletion and activation of oxidative stress [8].
Further studies have confirmed the role of PARP metabolism as an important mechanism in the development of endothelial dysfunction in cardiovascular pathologies caused by impaired carbohydrate metabolism - diabetes, and it has recently been confirmed that PARP may be involved in the development of diabetic polyneuropathy. Summarizing these data, we can make an assumption about the important role of PARP in ischemic heart disease, cerebrovascular diseases and diabetes [3, 5, 7, 8].
MW Elmlinger et al., using brain cell cultures (primary hippocampal neurons), in their study showed the inhibitory effect of Actovegin on oxidative stress processes. In neurons treated with increasing concentrations of tert-butyl hydroperoxide (> 0.2 mM), an increase in intracellular ROS levels was found (p < 0.001), but in the case of Actovegin in cultured neurons, a dose-dependent decrease in the severity of oxidative stress was noted after 10 days (p < 0.001 at concentrations > 0.3 µg/ml) [8]. In in vivo studies, the effect of Actovegin on the analyzed parameters in experimental diabetic polyneuropathy corresponded to the results obtained in this in vitro study [6, 7].
Actovegin® has a multifaceted effect by normalizing the consumption and use of oxygen, increasing the entry of glucose into cells, thereby restoring cellular metabolism [6, 9, 10]. Actovegin® enhances oxidative processes, shifting the balance of redox reactions towards oxidation, which helps to increase the content of high-energy phosphates, such as ATP and creatine phosphate. K. Schwabe showed that Actovegin® activates intracellular oxidative processes and accelerates not only energy, but also reserve metabolism, which in the case of heart disease is accompanied by increased accumulation of glycogen and potassium. These data were one of the first observations showing a direct positive effect of the drug Actovegin® on the metabolism of the brain and myocardium [5, 8].
Previous studies document that Actovegin® has an insulin-like effect through the activation of GLUT 1–4, stimulating cellular metabolism, increasing oxygen consumption and energy production. One of the constituent parts of Actovegin fractions is Inositol-Phosphate-Oligosaccharide (IFO-fraction), which, through the activation of cAMP and adenylate cyclase, promotes intracellular glucose utilization, stimulates the efficiency of O2 consumption and reduces the formation of lactates.
The effect of Actovegin on glucose transport into the cardiomyocyte is insulin-independent (does not affect insulin receptors), since it is realized through the direct activation of GLUT 1–4, so its effect persists even against the background of insulin resistance in patients with type 2 diabetes. At the same time, the IFO fraction, in synergy with superoxide dismutase and magnesium, promotes the inhibition of lipid peroxidation of cell membranes (membrane stabilizing effect) [4, 6–8, 10].
A number of clinical studies have shown that the use of the drug Actovegin® has a positive effect on cognitive functions in cardiovascular encephalopathies, improves psychological and behavioral reactions, and is most effective in mild and moderate cognitive impairment [3, 6].
Thanks to the development and introduction into clinical practice of new medical technologies, in particular positron emission tomography (PET), there is now a real possibility of quantitative assessment of myocardial perfusion in vivo, oxygen uptake, glucose utilization, FA, and contractility. PET allows a non-invasive way to study oxygen uptake, glucose and FA metabolism with the calculation of quantitative parameters in absolute values. [18F]-2-fluoro-2-deoxyD-glucose ([18F]FDG), a glucose analogue labeled with 18-fluorine, which is not metabolized and remains unchanged in the cell cytosol, is used as a natural marker of glucose uptake and metabolism in PET. . To quantify the metabolism of FFAs in the human myocardium, [18F]-thia-hepta-decanoic acid ([18F]TDA), a long-chain false FA labeled with 18-fluorine, the accumulation of which indicates β-oxidation of FFAs, is currently used as a natural marker. in the myocardium as the main source of energy in the myocardium. To assess oxidative metabolism in PET, a model was developed using [1-11C]-acetate as a marker of myocardial oxygen uptake [1, 4].
In Fig. Figure 5 shows examples of quantitative assessment of O2 utilization of glucose metabolism (Fig. 5A) and free fatty acids (Fig. 5B) before and after intravenous infusion of 1000 mg Actovegin. Actovegin promotes a 3-fold increase in O2 utilization with a simultaneous 6-7-fold increase in glucose uptake and a similar decrease in FFA metabolism. Such dynamics of oxidative metabolism indicate Actovegin’s stimulation of aerobic oxidation, the most beneficial source of macroenergy phosphates [4].
There is evidence that the effect of Actovegin on patients with coronary artery disease complicated by acute coronary insufficiency is multicomponent, in addition to improving myocardial metabolism, it has a positive effect on the rheological properties of blood: it reduces the aggregation activity of platelets, increases the mobility of erythrocytes, and reduces blood viscosity (through a hypoglycemic effect). At the same time, Actovegin promotes angiogenesis – the development of collateral circulation [9].
According to a number of authors, the use of Actovegin by patients on the first day of development helps restore the contractile function of the myocardium of the left ventricle of MI through improving the metabolism of cardiomyocytes, eliminates electrical heterogeneity, which overall manifests itself in a reduction in the frequency of complications and early hospital mortality [4, 5].
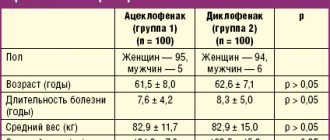
In our studies, the use of Actovegin (from 800 to 1200 mg intravenously) in the acute phase of MI in 49 patients against the background of thrombolysis and standard therapy contributed to a more effective prevention of the development of “reperfusion” syndrome (progression of pain syndrome, increase in episodes of ventricular arrhythmias, spread of the MI zone, increase in HF ). The comparison group consisted of 67 patients with AMI who underwent artificial thrombolysis without prophylactic intravenous administration of Actovegin. Analyzes of the clinical status and studies of the pumping activity of the heart before and after treatment by group are presented in the table.
As can be seen from the table, the reduction in the incidence of reperfusion syndrome to 18.4% in the study group of AMI patients compared to the control group of AMI patients (67 AMI patients) - 34%, was mediated by improving effective diastole (elimination of “contracture” of ischemic myocardium) . All parameters of blood flow through the mitral valve, characterizing the diastolic relaxation of the left ventricular myocardium, improved statistically significantly, which contributed to an effective increase in the ejection fraction of the left ventricle - an integral indicator of the pumping activity of the heart. EF in the study group during the administration of Actovegin increased statistically significantly (p < 0.05) by 8.4% (from 39.2 ± 5.5% to 46.6 ± 2.1%) compared with the control group of patients with AMI (from 39.1 ± 2.9% to 42.7 ± 3.1%).
More effective restoration of the metabolism of ischemic myocardium during the administration of Actovegin helps to minimize electrical heterogeneity, which is clinically manifested by a decrease in the incidence of ventricular arrhythmias: in the study group after treatment they occurred in 22.4% of cases, in the comparison group - in 32.8 %.
Similar results were obtained in other clinical observations [5].
Actovegin, as an antihypoxant and secondary antioxidant, when used in clinical practice, activates aerobic respiration of cells in a state of ischemia and metabolic failure, and has a systemic effect on the body (Fig. 6). The main pharmacological actions of Actovegin are increasing the efficiency of oxygen absorption and activating glucose transport, in particular in the cardiomyocyte. Activation of aerobic oxidation processes increases the energy potential of the myocardial cell. The listed effects of Actovegin are most pronounced in the hypoxic status of the heart muscle.
Thus, the development of cardiovascular disorders during ischemic episodes is accompanied by a set of pathophysiological events, the elimination of which requires an integrated pharmacological approach, rather than a simplified unidirectional effect. Multiple cardio- and neurotropic effects suggest a simultaneous modulating effect on various damaging pathological mechanisms (inflammation, apoptosis, oxidative stress, and many others).
As a biological agent with pleiotropic effects, Actovegin in its mechanisms of action (antioxidant, antihypoxic) corresponds to the concept of an integrative therapeutic approach.
The presented review and analysis of our own experience shows that Actovegin takes an active part in restoring the balance of cellular metabolism by correcting a number of pathophysiological processes that occur during the development of IHD. Actovegin has a cardioprotective effect on the cardiovascular functional block due to its anti-apoptotic and antioxidant effects, activates the mechanisms of glucose and oxygen utilization with the normalization of intracellular energy balance, which helps improve the pumping activity of the heart.