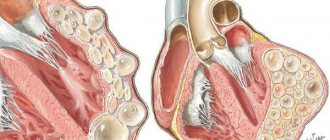
Insulin is a hormone produced by the beta cells of the islets of Langerhans in the pancreas. The name insulin comes from the Latin insula - island. Effects of insulin
Although insulin causes many effects in various tissues of the human body, its main effect is to stimulate the movement of glucose from the blood into cells, which leads to a decrease in blood glucose concentrations.
Other effects of insulin are stimulating the synthesis of glycogen from glucose in the liver and muscles, increasing the creation of fats and proteins, and suppressing the activity of enzymes that break down fats and proteins. Thus, insulin has an anabolic effect because it enhances the formation of fats and proteins while slowing down their breakdown.
The main effect of insulin is to enhance the transport of glucose across the cell membrane into the cell. There are no other hormones that lower blood glucose levels in the human body. The main effects of insulin occur in muscle and adipose tissue, which is why these tissues are called insulin-dependent. Blood glucose levels decrease when exposed to insulin and increase when exposed to the so-called. hyperglycemic hormones (glucagon, growth hormone, glucocorticoids).
Additional effects of insulin are an increase in the intensity of glycogen formation, a decrease in the formation of glucose in the liver, and an increase in the absorption by cells of amino acids necessary for protein synthesis. At the same time, insulin reduces the destruction of proteins and fats. Thus, the overall effect of insulin is anabolic - aimed at the formation of fat and muscle tissue.
Discovery of insulin
Pancreatic islets were discovered in 1869 by Paul Langerhans during a microscopic examination of the structure of the pancreas. In 1889, Oscar Malinowski in Germany, while removing a dog's pancreas, caused symptoms of diabetes mellitus. In 1921, F. Banting and C. Best isolated insulin from pancreatic islet cells, and D. Collip developed a method for its purification.
In 1922, insulin was first administered to a patient suffering from diabetes. Its therapeutic effect has shown that this type of therapy is the most effective. In subsequent years, the main efforts of scientists were aimed at organizing production in large quantities. In 1923, the Nobel Prize was awarded for the discovery and isolation of insulin. Subsequently, the amino acid structure of insulin was completely deciphered by F. Sanger.
pharmachologic effect
Insulin drugs affect all tissues and internal organs of the human body. But its “work” is most pronounced in its effect on muscle and fat tissue, the liver, metabolic processes occurring in the body, and digestion. Among the main functions of these drugs:
- regulation of carbohydrate metabolism due to stimulation of glucose penetration through the cell membrane, which promotes its conversion into glycogen and further utilization;
- increased glycogen content in muscle tissue;
- stimulation of the formation of peptides, glucosyltransferase, hexokinase enzyme, pyruvate dehydrogenase multienzyme complex;
- suppression of fat breakdown (lipolysis), which leads to a reduction in the amount of free fatty acids and reduces their entry into the systemic circulation;
- reduces the formation of glucose from fatty acids and amino acids, prevents the breakdown of glycogen (glycogenolysis);
- prevents the synthesis of ketone (acetate) bodies;
- slows down the process of converting amino acids into oxocarboxylic acids and so on.
The effectiveness of the drug depends on the characteristics of the body, muscle activity, blood flow speed at the injection site, the administered drug and its dose. The effect of a substance can be different not only in different people, but also in the same person, depending on his condition.
Insulin synthesis
In the islet cells of the pancreas, insulin is synthesized in several stages. At the first stage, the synthesis of the insulin precursor molecule, preproinsulin, occurs. At the second stage, the signal peptide is separated from the preproinsulin molecule, after which proinsulin is formed. After maturation, the final insulin molecule is formed. At the maturation stage, the C-peptide is separated from the proinsulin molecule, which has no biological effect. After separation of the C-peptide, the active form of insulin is formed.
Insulin is released into the blood when the level of glucose in the blood increases. Additionally, the regulation of insulin production is carried out by the autonomic nervous system. Insulin is destroyed in the liver and kidneys by the enzyme insulinase.
Release form
Insulin preparations go on sale in the form of solutions or suspensions, packaged in glass hermetically sealed bottles (5-10 ml). The top of the cork is rolled with an aluminum cap. For use in conjunction with a syringe pen, medicines are packaged in special cartridges (cases, cartridges).
For use in medical institutions, the drug can be in the form of a soluble white powder. It contains at least 3.1% sulfur. To introduce it into the body, it is diluted with special water for injection with the addition of hydrochloric acid, glycerin, and a solution of phenol (tricresol).
Insulin preparations
Currently, the pharmaceutical industry produces a significant number of insulin preparations with various biological effects. There are human, porcine, and bovine insulins. According to the degree of purification, traditional, monopeak, and monocomponent insulins are distinguished. Based on the duration of action, insulins are divided into short- and long-acting insulins. The latter are divided into medium-, long- and extra-long-acting insulins. There are also ultra-short insulins and depot insulins, which are released slowly from the subcutaneous tissue.
Selecting an insulin therapy regimen is a complex and very responsible undertaking. The success of achieving compensation for diabetes mellitus and, as a consequence, the patient’s quality of life depends on the correct choice of the form of insulin and its dosage regimen.
UZ "Mogilev City Emergency Hospital"
Main questions
- Carbohydrate ratio (calculation of the dose of short-acting insulin per meal).
- Dose of short-acting insulin for correction and insulin sensitivity factor.
- Time from insulin administration to the start of food intake (pause).
- Correction of high glycemic levels
Short-acting insulin (other names: short-acting, dietary, bolus, prandial insulin) ensures the absorption of carbohydrates that come with food. The injection of short-acting insulin is called an inulin bolus.
There are genetically engineered short-acting insulins and ultra-short-acting insulins (analogs).
It is necessary to understand the action of insulin: the time of onset of action, peak of action, duration of action (action of the main active part of the dose and “working off” of insulin).
Short genetically engineered insulins begin to act in 30-40 minutes, peak in 2-3 hours, the duration of the main active dose action is up to 4 hours, and the tail of the action is up to 5-6 hours.
Ultra-short insulins (analogues) begin to act 10-15 minutes after the injection, peak - 30-90 minutes after the injection, duration of active action - 2.5-3 hours, working out - up to 5 hours.
The action time of insulin can vary and depends on:
from the injection site (when injected into the stomach, insulin will act faster than when injected into the thigh);
time of day (insulin acts more slowly in the morning);
insulin doses (the larger the dose, the longer the insulin lasts);
physical activity and other factors.
Without understanding the action of insulin, diabetes compensation is impossible.
In a person with normal pancreatic function, the amount of insulin corresponds to the amount of carbohydrates that came from food. Those. The more carbohydrates a person ate, the more insulin was released. When using insulin therapy, you must follow the same principle: the amount of short-acting insulin administered should correspond to the amount of carbohydrates.
To do this you need:
1) be able to determine which foods contain carbohydrates and their quantity;
2) be able to calculate how many units of short-acting insulin should be administered per amount of carbohydrates eaten.
The required amount of insulin is determined using carbohydrate ratios. Carbohydrate ratio (CC) is the number of units of insulin that must be administered per amount of carbohydrates (the amount of carbohydrates is measured in XE or grams). That is, if your UC is 0.8 units per 1 xe and you want to eat 40 grams of carbohydrates (4 xe), then you need to enter 3 units of insulin (0.8 x 4 = 3.2). If you want to eat 50 grams of carbohydrates (5 XE), then you need to enter 4 units of insulin (0.8x5=4).
When prescribing insulin therapy, the initial dose of insulin is prescribed empirically by the doctor. In the hospital, doses are selected according to the hospital regime and hospital nutrition. If a person continues to follow a similar regimen (for example, older people), then he can remain on the same doses (provided he eats the same amount of carbohydrates and the same physical activity). But even at the same time, the need for insulin may change.
If a person wants to lead a more free lifestyle, he must learn to adjust the insulin dose using UC. Each person has their own Criminal Code! The CV of the same person changes during the day. In the morning, as a rule, the UC is higher (i.e., more insulin is needed per 1 XU); in the evening below, in the afternoon – average value. The Criminal Code may change due to the occurrence of other diseases, etc., as well as for no apparent reason.
When selecting a management company, the following conditions must be met:
1) fixed power mode:
eating food strictly at the right time after “working off” the previous dose of insulin;
strict carbohydrate counting (weigh portions);
simple food: porridge, boiled potatoes, boiled meat, fish, vegetables, fruits (foods with a high glycemic index are excluded, as well as complex foods: pancakes, dumplings, cheesecakes, etc.).
The best thing is the same menu for several days. It is very important that during the period of selecting insulin doses, the amount of carbohydrates eaten for the same amount of insulin does not change (since it will be impossible to correctly calculate the UC);
2) selection is carried out in a “sofa” mode (to exclude the influence of physical activity);
3) mandatory diary keeping. The following data is entered into the diary:
food (name, weight, amount of XE, time of meal);
time, dose and place of insulin administration;
time of blood glucose measurement;
4) frequent measurements of blood glucose levels: before the main meal (assessment of the adequacy of the previous dose) and before a snack. In the future, measurements are added 1 hour after eating;
5) odds are selected several days in advance using the same menu and mode;
6) absence of inflammatory diseases/stress;
7) it is impossible to select UC in the first 4-6 hours after hypoglycemia;
 The UC is calculated if the initial glucose level and the glucose level at the end of a given dose of insulin (4-5 hours after insulin administration) are within target levels;
The UC is calculated if the initial glucose level and the glucose level at the end of a given dose of insulin (4-5 hours after insulin administration) are within target levels;
9) it is possible to choose the right UC only if the doses of basal insulin are selected correctly and basal insulin does not lead to sharp fluctuations in glycemia OUTSIDE of meals.
If all of the above conditions are met, to calculate the UC, the dose of short-acting insulin that was administered before this meal must be divided by the number of XE that were eaten (main meal + snack).
Let's look at the selection of management companies for breakfast. Diary example
8.00 glucose 6.2 mmol/l
8.30 monoinsulin 6 units
9.00 BREAKFAST: Millet porridge, 200 g – 3 HE. Egg. Tea without sugar. Bread 1 piece - 1 XE.
11.00 2nd breakfast : Salad - 0.5 XE. Apple 1 XE.
13.40 glucose 8.2 mmol/l.
Initially and by the time insulin is released by lunch, glucose is within the target level, which means UC = 6 IU of insulin/5.5 XE (4 for breakfast + 1.5 for breakfast 2). UK = 1.1.
There are mathematical formulas for calculating the Criminal Code. For example, “Rule 2.61”: UK [U/XE] = (1.75× daily insulin dose) / body weight (kg). For example, a person weighing 90 receives 30 units of insulin analogues (short + long) per day. UK = 1.75x30/90 = 0.6 IU of insulin per 1 XE.
However, these formulas only work when the dose of insulin has already been selected and with certain reservations (they do not take into account changes in blood volume during the day, etc.).
You can calculate insulin doses using alternative coefficients: CR ( carbohydrate ratio ) - the number of grams of carbohydrates per 1 unit of insulin. CR is inversely proportional to UC.
For example, UK is 0.5 (for 1 XE we introduce 0.5 IU of insulin, XE = 10 grams of carbohydrates). We eat 50 grams of carbohydrates (5 XE), inject 2.5 units of insulin. CR in this case: 10/0.5 = 20. 50 grams of carbohydrates / CR = 50/20 = 2.5 units of insulin.
There are also mathematical formulas to calculate CR. For genetically engineered insulins “Rule 450”: CR [g/IU] = 450 / daily dose of insulin. For ultra-short insulin analogues “Rule 500”: CR [g/IU] = 500 / daily dose of insulin. For example, a person receives 40 units of insulin analogues (short+long) per day. CR =500:40=12.5. That is, if a person eats 12.5 g of carbohydrates, he needs to administer 1 unit of short-acting insulin, 25 - 2 units, if 50 g - 4 units. But, as with the calculation of the Criminal Code, one should not overestimate the importance of the formulas. All coefficients must be selected individually.
But the dose of short-term insulin before meals depends not only on the UK. For example, you have UC for breakfast = 1 (i.e., for 1 XE, enter 1 unit of insulin). For breakfast you eat 3 XE and inject 3 units of insulin. But yesterday before breakfast, glucose was 5.0, and today it is 12. This means that today 3 units of insulin will not be enough. Additional insulin must be administered to reduce glucose to the target level. This will be the insulin dose for correction.
The correction dose of insulin is the dose of insulin to reduce elevated glycemic levels when insulin is administered before meals.
To determine the dose for correction, you need to know your insulin sensitivity factor - ISF Insulin sensitivity factor - how much mmol/l reduces blood glucose levels by 1 unit of insulin. The FPI is also called the price per unit of insulin (PUI). The FCI, like the UC, may differ depending on the time of day and a number of factors (in the morning the FCI is usually low, i.e., in order to reduce glucose, more insulin must be administered in the morning than in the evening).
There are formulas for calculating the FFI. For ultra-short-acting insulins, FCI = 100/daily dose of insulin, for short-acting insulins, FCI = 83/daily dose of insulin. The daily dose of insulin is the sum of the doses of short-term insulin (including insulin for food and for correcting high glucose levels) and long-term insulin per day. Because The insulin dose can vary; you need to calculate the daily dose over several days (ideally, 2 weeks) and calculate the average daily dose.
For example, a person receives on average 20 units of short-acting insulin and 14 units of long-acting insulin per day, for a total of 34. 83:34 = 2.4. This means FCI = 2.4 and 1 unit of insulin will reduce glucose by 2.4 mmol.
However, again, these formulas are only suitable for determining the starting point from which we will start. You can find out your FCI only by repeated blood glucose measurements.
To check the FFI, you need to measure your blood glucose, administer 1 unit of short-acting insulin, eat nothing, exclude physical activity and check your blood glucose after 3-4 hours. In this case, the following conditions must be observed:
a) when checking the FFI, the initial glucose level should be initially high (at least 10 mmol/l).
b) there should be no active short-term insulin and active carbohydrates in the body. Those. in the morning we don’t eat breakfast, measure glucose, inject 1 unit of insulin, don’t eat, don’t move actively, measure glucose after 3-4 hours. If we check the FCI during the day, then we wait until the previous dose of short-acting insulin is completed (4-5 hours), measure glucose, administer 1 unit of insulin, do not eat, do not actively move, measure glucose after 3-4 hours.
If it is not possible or desirable to skip a meal, you can check the FCI by changing the dose for correction. But this must be done while taking a standard amount of carbohydrates in food. In this case, it is desirable that the Criminal Code is already known.
In general, the FFI is refined gradually over time during the process of measuring blood glucose.
Each person's Criminal Code and FCI are individual! They can only be determined experimentally with the help of ACCURATE CARBOHYDRATE COUNTING AND MULTIPLE GLUCOSE MEASUREMENTS!
To calculate the dose for correction, the difference between the existing and target blood glucose levels must be divided by the FCI. For example, blood glucose is currently 12, target level is 8, difference is 4. FFI 1. Correction dose = 4:1 = 4.
Let's go back to the example. UC for breakfast = 1, FCI = 1. For breakfast you are going to eat 3 HE. Glucose 12 mmol, target glucose level after meals 9, difference between existing and target blood glucose levels = 3 (12-9 = 3). Dose for correction: difference/FCI = 3:1 = 3. Dose for food = 3 UNITS. Insulin dose = 3 units for food + 3 units for correction = 6 units. So, for the same meal in the morning with a glucose of 5 mmol/l you will inject 3 units of insulin, with a glucose of 12 mmol/l - 6 units.
And then you need to check your glucose before insulin release (in this example, before lunch). If glucose is within the target level, then the dose was calculated correctly.
Is it enough to measure blood glucose only before meals and before the dose is worked out (in this example, before breakfast and lunch)? No. It is necessary to take measurements 1 hour after a meal (assessment of the food peak) and 2 hours after a meal (at the peak of insulin action). Measuring peak insulin shows whether the snack will fit. The point of the snack is to prevent hypoglycemia at the peak of insulin. But if glucose levels are high, then a snack is not needed. Measurement 1 hour after a meal shows the food (postprandial) peak (i.e. blood glucose level after the food has been absorbed). Ideally, the food peak is +1.5-3 mmol (up to 4 mmol) to the glucose level before meals.
The food peak is regulated by a pause. The pause is the time from the injection of insulin until you start eating.
The purpose of the pause is to achieve a match between the absorption profile of food and the action of insulin and, as a result, to keep glucose in the target range throughout the entire post-meal period. The pause is selected according to the food peak (measured with a glucometer 1-1.5 hours after eating). If we observe a high peak after an hour, but by the time insulin is released, glucose decreases to target values, then food has outpaced insulin.
The pause depends on:
1) glucose level (if the sugar is low, the pause is short or there is no pause at all, if it is high, the pause is longer, you need to let the insulin unfold, and then start eating). The principle is “lower sugar, less pause.”
Approximate pauses based on glucose levels can be seen in the table (the table is very arbitrary and is only a guideline for starting to select a pause, since pauses are very individual).
| Blood glucose (mmol/l) | Pause time | |
| For short insulin | For ultra-fast insulin | |
| < 6 | 15 minutes breakfast, 5 minutes lunch, no pause dinner | right before eating breakfast, during or after eating lunch and dinner |
| 6-8 | 20-30 minutes breakfast, 15 minutes lunch, 10 minutes dinner | 10 minutes breakfast, right before meals lunch and dinner |
| 8-12 | 30-40 minutes breakfast, 20-30 minutes lunch, 10-20 minutes dinner | 15 minutes breakfast, 10 minutes lunch and dinner |
| >12 | 40-50 minutes breakfast, 30-35 minutes lunch, 20-25 minutes dinner | 20 minutes breakfast, 15 minutes lunch and dinner |
2) time of day - in the morning the pause is usually longer, because In the morning, insulin unfolds more slowly;
3) the food you want to eat (if the food is “fast” with a high glycemic index, the pause is shorter, if the food is “slow” the pause is longer);
4) type of insulin : analogues act faster, the pause is shorter (0-15 minutes) or there is none at all;
5) individual characteristics of the organism. For example, with cicatricial changes in the esophagus or stomach, gastroparesis, impaired absorption of carbohydrates, enzyme deficiency of the pancreas, when food is absorbed slowly, the pause should be shorter. Otherwise, insulin will begin to act, but the food will not yet be absorbed. Sometimes in such cases it is necessary to administer insulin (especially ultra-short analogues) after meals (post-pause).
6) the presence of acute gastrointestinal diseases (for example, rotavirus infection), the presence of nausea and vomiting.
The pause is also selected individually! There may be a different pause for each time of day and product.
If by the end of the test the glucose is within the target level, and the postprandial peak is high, then the next day, with the same food and the same dose of insulin, we increase the pause by 5-10 minutes and check again. And so on several times until we reach the target postprandial level. It is necessary to ensure that the absorption time of carbohydrates coincides with the action time of insulin. Those. so that insulin does not begin to act earlier or later than carbohydrates are absorbed.
MAIN CONCLUSIONS:
- The correct dose of short-acting insulin administered with food is assessed by the glucose level before the next main meal (while working out the insulin dose).
- The correctness of the pause is assessed by the food peak.
- The dose of short-acting insulin is selected correctly if the glucose level returns to the original level 3-4 hours after eating.
- The pause is chosen correctly when the food peak is +1.5-3 mmol to the glucose level before meals.
In addition to administering short-acting insulin with food, short-acting insulin can also be administered to correct high glycemic levels without food.
For example, you measured your glucose level and it is 20. You want to reduce it to 12.
The dose of insulin to be administered is determined by the FCI. Your FCI is 2 (i.e. 1 unit of insulin reduces glucose by 2 mmol). Calculation of the insulin dose: the number of mmol of glucose by which to reduce / FCI. In this example, the glucose level needs to be reduced by 8 mmol (20-12 = 8), which means we divide 8 by the FCI and get 8:2 = 4 units of insulin must be administered.
But before you administer insulin, you need to figure out a few things:
Is there active insulin in the body (part of the previously administered bolus dose that has not yet completed its action)?
Are there active carbohydrates in the body and what kind (what causes the rise)?
Have you had previous hypoglycemia?
If the peak action of insulin has not occurred, there is no need to immediately administer insulin for correction. You need to wait and check your glucose after 1 hour. If it decreases, we continue to measure every hour until the insulin runs out. Perhaps there was simply a mismatch between the food and insulin profiles. This is especially important when selecting doses and pauses.
If you nevertheless administered a dose for correction, you do not need to inject additional insulin until it is completed. If you are not feeling well, you need to measure your blood glucose (preferably at least once an hour), then analyze the situation and draw conclusions. If you feel unwell, seek medical help.
Insulin doses can be changed in the event of acute diseases, exacerbation of chronic diseases, stress, etc.
Before physical activity, you often need to change your insulin dose. Hypoglycemia may occur during physical activity, so during exercise it is necessary to consume additional carbohydrates or reduce the dose of insulin. However, if physical activity occurs against the background of decompensation of the disease, glucose levels may increase. The issues of adjusting the dose of insulin during physical activity and acute illnesses are very well presented in the book “Sugar Man,” which we strongly recommend reading.