pharmachologic effect
Insulin glargine is an analogue of human insulin, obtained by recombination of DNA from bacteria of the species Escherichia coli (strains K12) and is characterized by low solubility in a neutral medium.
In patients with type 2 diabetes mellitus, the results of clinical studies have demonstrated a lower incidence of severe and/or confirmed hypoglycemia, as well as documented hypoglycemia occurring with clinical symptoms, when treated with Tujeo SoloStar compared to treatment with insulin glargine 100 U/ml.
The superiority of Tujeo SoloStar over insulin glargine 100 U/mL in reducing the risk of severe and/or documented nocturnal hypoglycemia was demonstrated in patients previously treated with oral hypoglycemic agents (23% risk reduction) or mealtime insulin (21% risk reduction) during the period from the 9th week to the end of the study, compared with treatment with insulin glargine 100 U/ml.
Insulin Tujeo SoloStar syringe pen 300 units/ml 1.5 ml N5 (Sanofi)
Hypersensitivity to insulin glargine or to any of the excipients of the drug. Age under 18 years (due to the lack of clinical data confirming the effectiveness and safety of the drug in children and adolescents). With caution: In pregnant women (the possibility of changes in insulin requirements during pregnancy and after childbirth), elderly patients (see sections “Pharmacokinetics”, “Pharmacodynamics”, “Method of administration and dosage” and “Special instructions”); patients with uncompensated endocrine disorders (such as hypothyroidism, insufficiency of the adenohypophysis and adrenal cortex); for diseases accompanied by vomiting or diarrhea; with severe stenosis of the coronary arteries or cerebral vessels; with proliferative retinopathy (especially if patients have not undergone photocoagulation; with renal failure; with severe liver failure (see section "Special instructions"). Pregnancy and lactation: Patients with diabetes mellitus should inform their doctor about the current or planned pregnancy. No randomized studies have been conducted controlled clinical studies on the use of the drug Tujeo SoloStar® in pregnant women. A large number of observations (more than 1000 pregnancy outcomes with retrospective and prospective observation) with post-marketing use of insulin glargine 100 U/ml showed the absence of any specific effects on the course and outcome of pregnancy , fetal or neonatal health. In addition, to evaluate the safety of insulin glargine and insulin isophane in pregnant women with pre-existing or gestational diabetes mellitus, a meta-analysis of eight observational clinical studies was conducted that included women who used insulin glargine 100 U/ml (n=331) and insulin isophane (n=371). This meta-analysis found no significant differences in maternal or neonatal safety between insulin glargine and isophane insulin during pregnancy. In animal studies, there was no direct or indirect evidence of the embryotoxic or fetotoxic effects of insulin glargine 100 U/ml when used in doses 6-40 times higher than recommended doses in humans. For patients with pre-existing or gestational diabetes mellitus, it is important to maintain adequate metabolic regulation throughout pregnancy to prevent adverse outcomes associated with hyperglycemia. If necessary, the use of Tujeo SoloStar® during pregnancy may be considered. Insulin requirements may decrease during the first trimester of pregnancy and generally increase during the second and third trimesters. Immediately after birth, the need for insulin decreases rapidly (the risk of hypoglycemia increases). In these conditions, careful monitoring of blood glucose concentrations is essential. Patients during breastfeeding may require adjustments to their insulin dosage regimen and diet.
Directions for use and doses
Toujeo SoloStar® units (insulin glargine 300 U/ml) refer only to Toujeo SoloStar and are not equivalent to other units expressing the potency of other insulin analogues. Tujeo SoloStar should be administered subcutaneously once a day at any time of the day, preferably at the same time. The drug Tujeo SoloStar, when administered once a day, allows for a flexible injection schedule: if necessary, patients can inject within 3 hours before or 3 hours after their usual time of administration.
Monitoring of blood glucose concentrations is recommended in all patients with diabetes mellitus.
Starting to use Tujeo SoloStar:
- Patients with type 1 diabetes mellitus. Tujeo SoloStar should be used once a day in combination with insulin administered during meals, and requires individual dose adjustment.
- Patients with type 2 diabetes mellitus. The recommended initial dose is 0.2 IU/kg 1 time per day, followed by individual dose adjustment.
Switching from the administration of insulin glargine 100 U/ml to the drug Tujeo SoloStar and, conversely, from the drug Tujeo SoloStar® to insulin glargine 100 U/ml
Insulin glargine 100 U/ml and Tujeo SoloStar are non-bioequivalent and not directly interchangeable.
- Switching from insulin glargine 100 U/mL to Toujeo SoloStar can be done on a unit-by-unit basis, but a higher dose of Toujeo SoloStar may be required to achieve the target plasma glucose concentration range.
- When switching from the use of Tujeo SoloStar to insulin glargine 100 U/ml, to reduce the risk of hypoglycemia, the dose should be reduced (by approximately 20%) followed by dose adjustment if necessary.
Close metabolic monitoring is recommended during and for the first few weeks after switching from one of these drugs to another.
Switching from other basal insulins to Tujeo SoloStar
When switching from a treatment regimen with intermediate- and long-acting insulins to a treatment regimen with Tujeo SoloStar, it may be necessary to change the dose of basal insulin and adjust the simultaneously administered hypoglycemic therapy (changes in the doses and timing of administration of short-acting insulins or fast-acting insulin analogues, or doses of non-insulin hypoglycemic drugs ).
- The transition from a single daily administration of basal insulin to a single daily administration of the drug Tujeo SoloStar can be carried out at the rate of one unit per unit of the previously administered dose of basal insulin.
- When switching from twice daily administration of basal insulin to a single administration of Tujeo SoloStar, the recommended initial dose of Tujeo SoloStar is 80% of the total daily dose of basal insulin, treatment of which is discontinued. Patients with high doses of insulin, due to the presence of antibodies to human insulin, may have an improved response to Toujeo SoloStar.
During the transition to Tujeo SoloStar and for several weeks after it, careful metabolic monitoring is recommended.
Insulin Tujeo SoloStar syringe pen 300U/ml 1.5ml N3 (Sanofi)
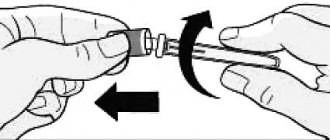
General recommendations Toujeo SoloStar® units (insulin glargine 300 U/ml) apply only to Toujeo SoloStar® and are not equivalent to other units expressing the potency of other insulin analogues. Tujeo SoloStar® should be administered subcutaneously once a day at any time of the day, preferably at the same time. The drug Tujeo SoloStar®, when administered once a day, allows for a flexible injection schedule: if necessary, patients can inject within 3 hours before or 3 hours after their usual time of administration. Target values for blood glucose concentrations, doses and timing of administration/administration of hypoglycemic drugs should be determined and adjusted individually. Dose adjustment may also be necessary, for example, if the patient changes his body weight, his lifestyle, changes in the timing of insulin administration, or other conditions that may increase the susceptibility to the development of hypo- or hyperglycemia (see "Special Instructions"). Any changes in insulin dosage should be made with caution and only under medical supervision. Tujeo SoloStar® is not the insulin of choice for the treatment of diabetic ketoacidosis. In this case, preference should be given to intravenous administration of short-acting insulin. Monitoring of blood glucose concentrations is recommended in all patients with diabetes mellitus. Initiation of use of the drug Tujeo SoloStar® Patients with type 1 diabetes mellitus. The drug Tujeo SoloStar® should be used once a day in combination with insulin administered during meals, and requires individual dose adjustment. Patients with type 2 diabetes mellitus. The recommended initial dose is 0.2 IU/kg 1 time per day, followed by individual dose adjustment. Switching from the administration of insulin glargine 100 U/ml to the drug Toujeo SoloStar® and, conversely, from the drug Toujeo SoloStar® to insulin glargine 100 U/ml Insulin glargine 100 U/ml and the drug Toujeo SoloStar® are not bioequivalent and are not directly interchangeable. — Switching from insulin glargine 100 U/mL to Toujeo SoloStar® can be done on a unit-by-unit basis, but a higher dose of Toujeo SoloStar® may be required to achieve the target plasma glucose concentration range. — When switching from the use of Tujeo SoloStar® to insulin glargine 100 U/ml, to reduce the risk of hypoglycemia, the dose should be reduced (by approximately 20%) followed by dose adjustment if necessary. Close metabolic monitoring is recommended during and for the first few weeks after switching from one of these drugs to another. Switching from other basal insulins to Tujeo SoloStar® When switching from a treatment regimen with intermediate- and long-acting insulins to a treatment regimen with Tujeo SoloStar®, you may need to change the dose of basal insulin and adjust the simultaneously administered hypoglycemic therapy (changes in doses and timing of administration of short-acting insulins action or fast-acting insulin analogues, or doses of non-insulin hypoglycemic drugs). — The transition from a single daily administration of basal insulin to a single daily administration of the drug Tujeo SoloStar® can be carried out at the rate of one unit per unit of the previously administered dose of basal insulin. — When switching from twice daily administration of basal insulin to a single administration of Tujeo SoloStar®, the recommended initial dose of Tujeo SoloStar® is 80% of the total daily dose of basal insulin, treatment of which is discontinued. Patients with high doses of insulin, due to the presence of antibodies to human insulin, may have an improved response to Tujeo SoloStar®. During the transition to Tujeo SoloStar® and for several weeks after it, careful metabolic monitoring is recommended. With improved metabolic control and the resulting increase in insulin sensitivity, additional dosage adjustments may be necessary. Adjustments to the dosage regimen may also be necessary, for example, if the patient's weight or lifestyle changes, if the timing of insulin dosing changes, or if other conditions occur that increase susceptibility to the development of hypo- and hyperglycemia. Transition from Tujeo SoloStar® to other basal insulins During the transition from Tujeo SoloStar® to other basal insulins and for several weeks thereafter, medical supervision and careful metabolic monitoring are recommended. It is recommended to refer to the instructions for use of the drug to which the patient is transferred. Mixing and dilution Tujeo SoloStar® cannot be mixed with any other insulin. Mixing leads to a change in the action profile of the drug Tujeo SoloStar® over time and causes precipitation. Tujeo SoloStar® cannot be diluted. Dilution may result in a change in the action profile of Toujeo SoloStar® over time. Special patient groups Children. The safety and effectiveness of Tujeo SoloStar® in children and adolescents under 18 years of age have not yet been established (see “Pharmacokinetics”). Elderly age. Tujeo SoloStar® can be used in elderly patients. Close monitoring of blood glucose concentrations is recommended, and the dose of insulin should be individualized. In elderly patients, progressive deterioration of renal function may lead to a permanent decrease in insulin requirements (see "Special Instructions", "Pharmacodynamics" and "Pharmacokinetics"). Kidney failure. Tujeo SoloStar® can be used in patients with renal failure. Close monitoring of blood glucose concentrations is recommended, and the dose of insulin should be individualized. In patients with renal failure, the need for insulin may be reduced due to a slowdown in insulin metabolism (see "Special Instructions", "Pharmacodynamics" and "Pharmacokinetics"). Liver failure. Tujeo SoloStar® can be used in patients with liver failure. Close monitoring of blood glucose concentrations is recommended, and the dose of insulin should be individualized. In patients with hepatic impairment, insulin requirements may be reduced due to decreased gluconeogenesis and slower insulin metabolism (see Pharmacodynamics, Pharmacokinetics, and Precautions). Method of administration The drug Tujeo SoloStar® is injected into the subcutaneous fat of the abdomen, shoulders or thighs. Injection sites should be rotated with each new injection within the recommended areas for drug administration. Tujeo SoloStar® is not intended for intravenous administration. The prolonged action of insulin glargine is observed only when it is administered into the subcutaneous fat. IV administration of the usual subcutaneous dose may cause severe hypoglycemia. Tujeo SoloStar® is not intended for administration using an insulin infusion pump. Tujeo SoloStar® is a clear solution and not a suspension, so resuspension is not required before use. Using the Toujeo SoloStar® syringe pen, you can administer doses from 1 to 80 units per injection in increments of 1 unit: - the dose counter of the Toujeo SoloStar® syringe pen shows the number of units of the Toujeo SoloStar® drug that will be administered. The Tujeo SoloStar® syringe pen was specially designed for the Tujeo SoloStar® drug, so no additional dose recalculation is required; — the drug Tujeo SoloStar® should never be removed from the pen cartridge into the syringe (see “Special instructions”); - Needles cannot be reused. A new sterile needle should be attached before each injection. Reusing needles increases the risk of needle blockage, which can lead to underdose or overdose. In addition, using a new sterile needle for each injection minimizes the risk of contamination and infection; - in case of needle blockage, the patient must follow the instructions indicated in step 3 (see Instructions for using the Tujeo SoloStar® syringe pen). To avoid the possible transmission of blood-borne diseases, insulin pens should not be used by more than one patient, even if the needle is changed. For proper use of the Tujeo SoloStar® syringe pen, see the Instructions for use of the Tujeo SoloStar® syringe pen. In order to exclude the possibility of erroneous (accidental) administration of another type of insulin instead of Tujeo SoloStar®, you should always check the label on the syringe pen before each injection (on the label of the syringe pen Tujeo SoloStar® con is highlighted with a honey-golden background). The period of use of the drug in a disposable syringe pen Tujeo SoloStar® after the first use is 4 weeks, when stored in a place protected from light. It is recommended to indicate the date of first use on the pen label. Instructions for using the Tujeo SoloStar® syringe pen (insulin glargine 300 IU/ml) The Tujeo SoloStar® syringe pen contains insulin glargine at a concentration of 300 IU/ml. 1. Never reuse a needle. If the needle is reused, because the needle may become clogged, the patient may not receive the dose he or she needs (underdosing) or may receive too much of the dose (overdosing). 2. Never use a syringe to extract insulin from a pen. In this case, the patient may receive too much insulin. The scale on most insulin syringes is only for non-concentrated insulin. Important information 1. Do not share a pen at the same time with other people, even if the needle is replaced. The patient may get a serious infection from other people or transmit a serious blood-borne infection to them. 2. Never use a syringe pen if it is damaged or the patient is not sure that it is working properly. 3. Always perform a safety test. 4. Always carry a spare pen and spare needles in case they get lost or become faulty. 5. Before using a syringe pen, check with your healthcare professional on how to properly administer a subcutaneous injection. 6. If the patient has vision problems, he may require the assistance of others who can follow all the recommendations in this manual for using the Tujeo SoloStar® syringe pen. Before using the pen, you should read all instructions. If the patient does not follow all the recommendations, he may receive either too much or too little insulin. The patient will additionally need: a new sterile needle (see Step 2), a wipe moistened with alcohol, and a puncture-resistant container for used needles and syringes.
special instructions
Patients must have skills in self-control of diabetes mellitus, including monitoring blood glucose concentrations, as well as adhere to the correct technique for performing subcutaneous injections and be able to stop the development of hypoglycemia and hyperglycemia. Insulin therapy requires constant vigilance regarding the possibility of developing hyperglycemia or hypoglycemia.
In case of insufficient control of the concentration of glucose in the blood, as well as in the presence of a tendency to the development of hypo- or hyperglycemia, before proceeding with the correction of the dosage regimen, you should check the accuracy of the prescribed treatment regimen, compliance with the instructions regarding the drug administration sites, the correctness of the subcutaneous injection technique and handling the SoloStar syringe pen, and also take into account the possibility of all other factors that can cause such a condition.
Storage conditions
In a place protected from light, at a temperature of 2–8 °C (do not freeze). Keep out of the reach of children.
When storing Tujeo SoloStar in the refrigerator (unopened/prior to use), you must ensure that the packages of syringe pens do not come into direct contact with the freezer compartment or frozen foods, because the drug should not be frozen. If insulin has been frozen, it cannot be used and the pen should be discarded.
Used SoloStar syringe pens should be stored at a temperature not exceeding 30°C, protected from exposure to light and heat. The period of use of the drug in a disposable syringe pen Tujeo SoloStar after the first use is 4 weeks, when stored in a place protected from light. It is recommended to indicate the date of first use on the pen label.