What is Motilium
The active component of the drug is domperidone, a substance that can quickly suppress nausea, normalize peristalsis, stool, and relieve signs of indigestion. The product is produced in several pharmaceutical forms:
- film-coated tablets: white, biconvex, containing 10 mg of domperidone, packaged in blisters;
- non-coated sublingual tablets with mint flavor;
- syrup for children: a sweetish, thick, white, water-based liquid with a dosage of domperidone 1 mg per 1 ml, sold in glass bottles with a measuring syringe.
The tablet medicine contains auxiliary compounds: starch, povidone, stabilizers.
For what diseases is Motilium needed?
The medicine helps restore well-being in the following pathologies:
- stomach ulcer;
- atonic bowel dysfunction;
- flatulence;
- hypokinetic dyskinesia of the gallbladder;
- epigastric pain;
- nausea, vomiting due to food or chemical intoxication;
- digestive disorders due to taking medications, radiation therapy;
- spontaneous constipation;
- frequent regurgitation in children.
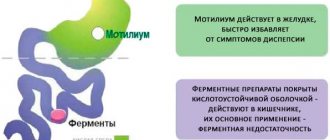
The drug moderately stimulates intestinal peristalsis, normalizing gastric functions and eliminating signs of dyspepsia. Domperidone suppresses dopamine activity, producing a mild antipsychotic effect. But, unlike other drugs, it does not lead to autonomic disorders.
The medicine quickly penetrates the blood through the mucous membranes and interacts with its proteins. After administration, Motilium cleanses the stomach cavity, intestinal lumen, binds and neutralizes toxic substances. It is completely excreted from the body with intestinal contents after 12–24 hours, without accumulating in tissues.
MOTILIUM suspension for oral administration 1 mg/ml bottle 100 ml
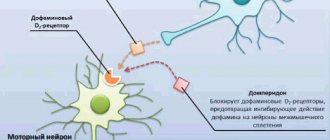
Motilium is an antiemetic. Pharmacodynamics Domperidone is a dopamine antagonist that, like metoclopramide and some antipsychotics, has antiemetic properties. However, unlike these drugs, domperidone does not penetrate the blood-brain barrier well. The use of domperidone is rarely accompanied by extrapyramidal side effects, especially in adults, but domperidone stimulates the release of prolactin from the pituitary gland. Its antiemetic effect may be due to a combination of peripheral (gastrokinetic) action and antagonism of dopamine receptors in the chemoreceptor trigger zone. When administered orally, domperidone increases the duration of antral and duodenal contractions, accelerates gastric emptying and increases lower esophageal sphincter pressure in healthy people. Domperidone has no effect on gastric secretion. Pharmacokinetics Domperidone is rapidly absorbed when administered orally on an empty stomach, with peak plasma concentrations occurring within approximately 1 hour. The low absolute bioavailability of oral domperidone (approximately 15%) is due to extensive first-pass metabolism in the intestinal wall and liver. Domperidone should be taken 15-30 minutes before meals. Hypoacidity of gastric juice reduces the absorption of domperidone. When taking the drug after a meal, it takes longer to achieve maximum absorption and the area under the curve (AUC) increases slightly. When taken orally, domperidone does not accumulate or induce its own metabolism. The maximum plasma concentration 90 minutes after oral administration at a dose of 30 mg per day for 2 weeks was 21 ng/ml, almost no different from the maximum concentration after a single dose (18 ng/ml). Domperidone is 91-93% bound to plasma proteins. Concentrations of domperidone in breast milk of lactating women are 4 times lower than the corresponding concentrations in plasma. The drug is metabolized in the liver by hydroxylation and N-dealkylation. Excretion in urine and feces accounts for 31 and 66% of the oral dose, respectively. The excretion of the drug unchanged is a small percentage (10% in feces and approximately 1% in urine). The plasma half-life after taking a single dose is 7-9 hours in healthy people, but is prolonged in patients with severe renal failure (20.8 hours).
How to take Motilium tablets
Adults are recommended to take 1 pill three times a day, 15–20 minutes before meals. The coated tablets are washed down with cool or warm water. Sublingual - placed under the tongue until completely dissolved. If necessary, the single dose is increased 2–3 times. The maximum number of tablets per day is 8 pcs. It is allowed to take the medicine before bedtime.
For children, treatment with Motilium in tablets is recommended from 6–7 years of age: 1 tablet 2–3 times a day. For body weights less than 35 kg, it is recommended to use a suspension of the drug.
Motilium®
Anticholinergic drugs can neutralize the effect of MOTILIUM. The oral bioavailability of MOTILIUM decreases after previous administration of cimetidine or sodium bicarbonate. You should not take antacid and antisecretory drugs simultaneously with MOTILIUM, as they reduce its bioavailability after oral administration (see section “Special Instructions”).
The main role in the metabolism of domperidone is played by the CYP3A4 isoenzyme. In vitro studies and clinical experience indicate that concomitant use of drugs that significantly inhibit this isoenzyme may cause increased plasma concentrations of domperidone. Strong CYP3A4 inhibitors include:
— Azole antifungals, such as fluconazole*, itraconazole, ketoconazole* and voriconazole*;
— Macrolide antibiotics, for example clarithromycin* and erythromycin*;
- HIV protease inhibitors, such as amprenavir, atazanavir, fosamprenavir, indinavir, nelfinavir, ritonavir and saquinavir;
- Calcium antagonists, such as diltiazem and verapamil;
— Amiodarone*;
- Aprepitant;
- Nefazodone.
(Drugs marked with an asterisk also prolong the QTc interval (see section “Contraindications”)).
In a number of studies of the pharmacokinetic and pharmacodynamic interactions of domperidone with oral ketoconazole and oral erythromycin in healthy volunteers, these drugs have been shown to significantly inhibit the primary metabolism of domperidone by the CYP3A4 isoenzyme.
With simultaneous administration of 10 mg of domperidone 4 times a day and 200 mg of ketoconazole 2 times a day, there was an increase in the QTc interval by an average of 9.8 ms during the entire observation period, at certain points the changes varied from 1.2 to 17.5 ms. With simultaneous administration of 10 mg of domperidone 4 times a day and 500 mg of erythromycin 3 times a day, there was an increase in the QTc interval by an average of 9.9 ms during the entire observation period, at certain points the changes varied from 1.6 to 14.3 ms. In each of these studies, the Cmax and AUC of domperidone were increased approximately threefold (see section "Contraindications").
It is currently unknown how elevated plasma concentrations of domperidone contribute to changes in the QTc interval.
In these studies, domperidone monotherapy (10 mg four times daily) prolonged the QTc interval by 1.6 ms (ketoconazole study) and 2.5 ms (erythromycin study), whereas ketoconazole monotherapy (200 mg twice daily) and erythromycin (500 mg three times daily) led to a prolongation of the QTc interval by 3.8 and 4.9 ms, respectively, during the entire observation period.
In another multiple-dose study in healthy volunteers, there was no significant prolongation of the QTc interval during inpatient domperidone monotherapy (40 mg four times daily, total daily dose 160 mg, 2 times the recommended maximum daily dose). However, plasma concentrations of domperidone were similar to those in studies of the interaction of domperidone with other drugs.
Theoretically, since MOTILIUM® has a gastrokinetic effect, it could influence the absorption of concomitantly administered oral drugs, in particular sustained-release or enteric-coated drugs. However, the use of domperidone in patients taking paracetamol or digoxin did not affect the blood levels of these drugs.
MOTILIUM® can be taken simultaneously with:
- neuroleptics, the effect of which it does not enhance;
- with dopaminergic receptor agonists (bromocriptine, L-dopa), since it inhibits their unwanted peripheral effects, such as digestive disorders, nausea and vomiting, without affecting their central effects.
Side effects of Motilium
The drug increases the synthesis of the hormone prolactin. In some cases, this can cause a short-term disruption of the menstrual cycle and provoke the development of gynemastia. Occasionally during treatment the following are possible:
- decreased appetite;
- dryness or unpleasant taste in the mouth;
- increased drowsiness;
- increased spasms in the intestines.
If any undesirable symptoms occur, you should consult your doctor.
Motilium Suspension, bottle, 100 ml, 1 mg/ml, for oral administration, for children
special instructions
When using Motilium together with antacid or antisecretory drugs, the latter should be taken after and not before meals, i.e.
they should not be taken simultaneously with Motilium. Motilium oral suspension contains sorbitol and is not recommended for use in patients with sorbitol intolerance.
Use in children
Motilium in rare cases can cause neurological side effects (see section "Side effects"). The risk of neurological side effects is higher in young children because metabolic functions and the blood-brain barrier are not fully developed in the first months of life. In this regard, you should very accurately calculate the dose of Motilium for newborns, children of the first year of life and children of early preschool age and strictly adhere to this dose (see section “Method of administration and doses”). Neurological adverse effects can be caused by overdose of the drug in children, but other possible causes of such effects must be taken into account.
Use for diseases of the cardiovascular system
Some epidemiological studies have shown that the use of domperidone may be associated with an increased risk of serious ventricular arrhythmias or sudden coronary death (see section "Side effects"). The risk may be more likely in patients over 60 years of age and in patients taking the drug in daily doses of more than 30 mg. Patients over 60 years of age should consult a doctor before taking Motilium (see section “Dosage and Administration”).
The use of domperidone and other drugs that lead to prolongation of the QTc interval is not recommended in patients with existing conduction disorders, in particular, prolongation of the QTc interval, and in patients with severe electrolyte imbalance (hypokalemia, hyperkalemia, hypomagnesemia) or with bradycardia, or in patients with concomitant heart diseases such as congestive heart failure. As is known, against the background of electrolyte imbalance and bradycardia, the risk of arrhythmias increases.
If signs or symptoms appear that may be associated with cardiac arrhythmia, Motilium therapy should be discontinued and a physician should be consulted.
Use for kidney diseases.
Since a very small percentage of the drug is excreted unchanged by the kidneys, single dose adjustment is not required in patients with renal failure. However, when re-prescribing Motilium, the frequency of use should be reduced to one or two times a day, depending on the severity of renal dysfunction (see section “Dosage and Administration”). During long-term therapy, patients should be monitored regularly.
Potential for drug interactions
The main route of metabolism of domperidone is via CYP3A4. In vitro data and human studies indicate that concomitant use of drugs that significantly inhibit this enzyme may be associated with increased plasma concentrations of domperidone. The combined use of domperidone with potent CYP3A4 inhibitors, which have been shown to cause prolongation of the QT interval, is contraindicated (see "Contraindications").
Caution is advised when coadministering domperidone with strong CYP3A4 inhibitors that do not prolong the QT interval, such as indinavir, and patients should be closely monitored for signs or symptoms of adverse reactions (see Interactions with Other Drugs).
Caution is advised when coadministering domperidone with drugs known to cause QT prolongation, and patients should be closely monitored for signs or symptoms of cardiovascular adverse reactions.
Examples of such medicines:
• class IA antiarrhythmics (eg disopyramide, quinidine);
• Class III antiarrhythmics (eg, amiodarone, dofetilide, dronedarone, ibutilide, sotalol);
• certain antipsychotics (eg haloperidol, pimozide, sertindole);
• certain antidepressants (eg citalopram, escitalopram);
• certain antibiotics (eg levofloxacin, moxifloxacin);
• certain antifungals (eg pentamidine);
• certain antimalarials (eg halofantrine);
• certain gastrointestinal medicines (eg dolasetron);
• certain anticancer drugs (eg toremifene, vandetanib);
• some other medicines (eg bepridil, methadone).
If the medicine has become unusable or the expiration date has expired, do not pour it into wastewater or onto the street! Place the medication in a bag and place it in the trash. These measures will help protect the environment!
When is Motilium contraindicated?
It is prohibited to use the medicine:
- in case of danger of perforation of a gastric ulcer, development of internal bleeding;
- in case of intestinal obstruction;
- in case of dysfunction, neoplasms in the pituitary gland;
- prolactinoma;
- severe kidney or liver damage;
- individual intolerance to any of the components of the drug.
If you are allergic to Motilium, itching, hyperemia, rash, lacrimation, and swelling of the mucous membranes are likely to occur. In such cases, discontinuation of therapy and replacement of the drug with another is required.
How does Motilium interact with other drugs?
During the period of using Motilium, it is advisable to stop using antacids and proton pump inhibitors - drugs that reduce the acidity of gastric juice. Simultaneous use slows down the action of Motilium and reduces its absorption.
Antimycotic drugs and drugs from the macrolide group inhibit the metabolism of domperidone. This is important to consider in cases of impaired renal and liver function.
When undergoing treatment with Ketoconazole, Azithromycin, Erythromycin and inhibitors of the CYP3A4 isoenzyme, Motilium should be discontinued. Otherwise, the risk of heart failure increases.
Motilium for kids
Release form, composition and packaging
The suspension for oral administration is homogeneous, white. 5 ml domperidone 5 mg. Excipients: sodium saccharin, microcrystalline cellulose, sodium carboxymethylcellulose, sorbitol, methyl parahydroxybenzoate, propyl parahydroxybenzoate, sodium hydroxide, polysorbate, purified water.
Clinical and pharmacological group: Centrally acting antiemetic drug that blocks dopamine receptors.
pharmachologic effect
Antiemetic drug, stimulant of gastrointestinal motility. Domperidone is a dopamine antagonist that, similar to metoclopramide and some antipsychotics, has antiemetic properties. However, unlike these drugs, domperidone penetrates the BBB poorly. Domperidone is rarely associated with extrapyramidal side effects, especially in adults, but domperidone stimulates the release of prolactin from the pituitary gland.
The antiemetic effect may be due to a combination of peripheral (gastrokinetic) action and antagonism of dopamine receptors in the chemoreceptor trigger zone. When taken orally, domperidone increases the duration of antral and duodenal contractions, accelerates gastric emptying - the release of liquid and semi-solid fractions in healthy people and solid fractions in patients when this process has been slowed down, and increases the pressure of the sphincter of the lower esophagus in healthy people. Domperidone has no effect on gastric secretion.
Pharmacokinetics
Suction
After oral administration on an empty stomach, domperidone is rapidly absorbed from the gastrointestinal tract. Cmax in blood plasma is achieved within approximately 1 hour. The low absolute bioavailability of domperidone when taken orally (approximately 15%) is due to extensive primary metabolism in the intestinal wall and liver. Although in healthy people the bioavailability of domperidone increases when taken after a meal, patients with gastrointestinal complaints should take domperidone 15-30 minutes before meals. Hypoacidity of gastric juice reduces the absorption of domperidone. When taking the drug after a meal, it takes longer to reach Cmax and the AUC increases slightly.
Distribution
When taken orally, domperidone does not accumulate or induce its own metabolism. After taking domperidone for 2 weeks at a dose of 30 mg/day, Cmax in blood plasma 90 minutes after the last dose was 21 ng/ml and was almost the same as after taking the first dose (18 ng/ml). Plasma protein binding - 91-93%. Concentrations of domperidone in breast milk of lactating women are 4 times lower than the corresponding concentrations in blood plasma.
Metabolism
Domperidone is metabolized in the liver by hydroxylation and N-dealkylation. In in vitro drug metabolism studies using diagnostic inhibitors, it was found that isoenzyme 3A4 is the main isoenzyme of the cytochrome P450 system involved in the process of N-dealkylation of domperidone, while isoenzymes CYP3A4, CYP1A2 and CYP2E1 are involved in the aromatic hydroxylation of domperidone.
Removal
Excretion in urine and feces is 31% and 66% of the dose taken, respectively. It is excreted unchanged in feces (10%) and urine (approximately 1%). T1/2 from blood plasma after taking a single dose in healthy volunteers is 7-9 hours.
Pharmacokinetics in special clinical situations
In patients with severe renal failure (serum creatinine level more than 6 mg/dL), T1/2 of domperidone increases from 7.4 hours to 20.8 hours, but plasma concentrations of the drug are lower than in healthy volunteers.
Indications
- a complex of dyspeptic symptoms, often associated with delayed gastric emptying, gastroesophageal reflux, esophagitis (a feeling of fullness in the epigastrium, a feeling of bloating, pain in the upper abdomen, belching, flatulence, nausea, vomiting, heartburn and regurgitation);
- nausea and vomiting of functional, organic, infectious origin caused by radiotherapy, drug therapy or diet disorders;
- nausea and vomiting caused by dopamine agonists when used in Parkinson's disease (such as L-dopa and bromocriptine);
- regurgitation syndrome, cyclic vomiting, gastroesophageal reflux and other gastric motility disorders in children.
Dosage regimen
Film-coated tablets are indicated for adults and children weighing more than 35 kg. Lozenges are indicated for adults and children over 5 years of age. In pediatric practice (especially children under 5 years of age), it is recommended to use Motilium in the form of a suspension.
For chronic dyspepsia, adults and children are prescribed 10 mg 3 times a day 15-30 minutes before meals and, if necessary, before bedtime. The maximum daily dose is 80 mg.
If necessary, for adults and children over 12 years of age, the dose can be doubled.
For children , the drug in the form of a suspension is prescribed at the rate of 2.5 ml/10 kg of body weight (which corresponds to 250 mcg/kg of body weight) 3 times a day before meals and, if necessary, before bedtime. If necessary, the indicated dose can be doubled (except for children under 1 year of age). The maximum daily dose is 2.4 mg/kg body weight, but not more than 80 mg. For nausea and vomiting, adults and children over 12 years of age are prescribed 20 mg 3-4 times a day before meals and at bedtime. The maximum daily dose is 80 mg.
Children aged 5 to 12 years are prescribed 10 mg 3-4 times a day before meals and at bedtime. The drug in the form of a suspension is prescribed at the rate of 5 ml/10 kg of body weight (which corresponds to 500 mcg/kg of body weight) 3-4 times a day before meals and before bedtime. This dose is achieved by filling the pipette twice. The maximum daily dose is 2.4 mg/kg body weight, but not more than 80 mg.
In case of renal failure, it is recommended to increase the interval between taking the drug. Because Since a very small percentage of the drug is excreted unchanged by the kidneys, there is hardly any need to adjust a single dose in patients with renal failure. However, when re-prescribed, the frequency of administration should be reduced to 1-2 times a day, depending on the severity of renal failure, and a dose reduction may also be required.
Rules for using the suspension
The bottle of suspension should be shaken before use. The suspension is supplied in child-resistant packaging, so the bottle should be opened as follows:
- Press down on the plastic cap of the bottle while turning it counterclockwise.
- Remove the unscrewed cap.
- Remove the pipette from the case (supplied only with the 100 ml bottle).
- Holding the lower ring in place, raise the upper one to the mark corresponding to the child’s weight (in kg).
- Holding the lower ring, remove the filled pipette from the bottle.
- After use, rinse the pipette with water, place the empty pipette back in the case and close the bottle.
Rules for the use of lozenges
Lozenges are available in blister packs. Since the tablets are quite fragile, they should not be pressed through the foil to avoid damage. In order to remove a tablet from the blister, take the foil by the edge and completely remove it from the cell in which the tablet is located. Then gently press down from below and remove the tablet from the package. The tablet should be placed on the tongue. Within a few seconds it will disintegrate on the surface of the tongue and can be swallowed with saliva without washing it down with water.
Side effect
- From the digestive system: rarely – gastrointestinal disorders; in isolated cases - transient intestinal spasms.
- From the central nervous system: extrapyramidal symptoms (very rarely - in children; in isolated cases - in adults); completely reversible and disappear after cessation of treatment. If the BBB is insufficiently developed (for example, in children under 1 year of age) or its functions are impaired, the possibility of neurological side effects cannot be completely excluded.
- From the endocrine system: hyperprolactinemia is possible, rarely leading to galactorrhea, gynecomastia, amenorrhea. Allergic reactions: rarely - rash, urticaria.
Contraindications
- gastrointestinal bleeding;
- mechanical obstruction or perforation, in which stimulation of the motor function of the stomach can be dangerous;
- prolactin-secreting tumor of the pituitary gland (prolactinoma);
- simultaneous use of oral forms of ketoconazole;
- hypersensitivity to the components of the drug.
Pregnancy and lactation
There is insufficient data on the use of Motilium during pregnancy. To date, there is no evidence of an increased risk of developmental defects in humans. However, the use of Motilium during pregnancy (especially in the first trimester) is possible only in cases where the expected benefit of therapy for the mother outweighs the potential risk for the fetus.
In women, the concentration of domperidone in breast milk is 10-50% of the corresponding concentration in plasma and does not exceed 10 ng/ml. The total amount of domperidone excreted into breast milk is less than 7 mcg/day when using the maximum permissible doses. It is unknown whether this level has a negative effect on newborns. Therefore, if it is necessary to use Motilium during lactation, breastfeeding should be stopped.
Use for liver dysfunction
Motilium should be prescribed with caution to patients with liver failure, given the high degree of metabolism of domperidone in the liver.
Use for renal impairment
How can I replace Motilium?
The most famous analogues and generics of the drug:
- Domrid;
- Domidon;
- Domperidone Hexal;
- Motinorm;
- Motoricum;
- Motilak;
- Peridonium.
You should not change the medicine to an alternative one on your own. In addition to differences in dosage, some of them have a wider list of contraindications; not all are approved for use in pediatrics.