Composition and form of the product
Nexium is produced in the form of tablets, extended-release capsules or lyophilisate in vials. The active substance in it is esomeprazole. Its content in various forms of the drug is:
- In tablets - 20 or 40 mg.
- In capsules - 10 mg.
- In bottles with lyophilisate – 40 mg.
Other auxiliary components that were used in the manufacture of the products are indicated in the manufacturer's instructions.
Action of the medicine
The action of zomeprazole is aimed at inhibiting the production of hydrochloric acid by the glands of the digestive organ. After taking Nexium tablets, the pH level of the stomach is quickly normalized. In this case, the indicators are maintained for 3–17 hours. In the future, a cumulative effect is ensured.
By providing a sustainable decrease in acidity, the medicine creates optimal conditions for the destruction of the bacterium Helicobacter pylori, which provokes the development of gastritis and peptic ulcers. In combination with antibiotics Nexium, the instructions focus on this, it allows you to destroy the pathogenic microorganism in almost a week. Patients with reflux esophagitis can get rid of unpleasant symptoms after a 4-week course of treatment. When using the drug, the risks of relapses and complications are significantly reduced.
Instructions for use NEXIUM
Adults and children over 12 years old
Gastroesophageal reflux disease
For the treatment of erosive reflux esophagitis
Nexium is prescribed in a single dose of 40 mg 1 time/day for 4 weeks. An additional 4-week course of therapy is recommended in cases where, after the first course, there is no cure for esophagitis or the symptoms of the disease persist.
With long-term maintenance therapy after treatment of erosive reflux esophagitis in order to prevent relapse
the drug is prescribed 20 mg 1 time/day.
For symptomatic treatment of gastroesophageal reflux disease without esophagitis
the drug is prescribed in a dose of 20 mg 1 time/day. If symptoms do not disappear after 4 weeks of treatment, the patient should be further examined. After eliminating the symptoms, you can switch to the “as needed” regimen of taking the drug, i.e. take Nexium 20 mg 1 time/day if symptoms occur until they subside. For patients taking NSAIDs who are at risk of developing gastric or duodenal ulcers, as-needed treatment is not recommended.
Adults
Peptic ulcer of the stomach and duodenum
As part of combination therapy for the eradication of Helicobacter pylori, as well as for the treatment of duodenal ulcers associated with Helicobacter pylori and for the prevention of relapses of peptic ulcers associated with Helicobacter pylori
in patients with peptic ulcer, Nexium is prescribed in a single dose of 20 mg, amoxicillin - 1 g, clarithromycin - 500 mg. All medications should be taken 2 times a day for 1 week.
Patients taking NSAIDs for a long time
For the healing of stomach ulcers associated with NSAID use,
Nexium is prescribed in a dose of 20 mg or 40 mg 1 time / day. The duration of treatment is 4-8 weeks.
For the prevention of gastric and duodenal ulcers associated with taking NSAIDs,
Nexium is prescribed in a dose of 20 mg or 40 mg 1 time / day.
In conditions characterized by pathological hypersecretion, incl. Zollinger-Ellison syndrome and idiopathic hypersecretion
Nexium is prescribed at an initial dose of 40 mg 2 times a day. In the future, the dose is selected individually, the duration of treatment is determined by the clinical picture of the disease. There is experience using the drug in doses up to 120 mg 2 times a day.
When prescribing the drug to patients with impaired renal function
no dose adjustment is required.
The drug is used with caution in patients with severe renal failure
due to limited clinical experience with its use in this category of patients.
When prescribing Nexium to patients with mild or moderate liver failure
no dose adjustment is required.
For patients with severe liver failure,
the maximum dose is 20 mg/day.
Elderly patients
no dosage adjustment is required.
Teenagers over 12 years of age
:
- treatment of erosive reflux esophagitis 40 mg once daily for 4 weeks. An additional 4 weeks of therapy is necessary for patients whose esophagitis has not resolved or symptoms persist:
- long-term maintenance therapy in patients after healing of erosive reflux esophagitis to prevent relapse 20 mg once a day;
- symptomatic treatment of gastroesophageal reflux disease 20 mg once daily in patients without esophagitis. If after 4 weeks it is not possible to achieve control of symptoms, the patient should be further examined. Once symptoms resolve, subsequent control can be achieved with 20 mg once daily;
- Treatment of duodenal ulcer associated with Helicobacter pylori When choosing an appropriate combination therapy, the official recommendations of the competent authorities regarding bacterial resistance and duration (usually 7 days, but not more than 24 days) are taken into account under the supervision of a specialist.
With a body weight of 30-40 kg:
- combination with two antibiotics. Nexium 20 mg, amoxicillin 750 mg and clarithromycin 7.5 mg/kg body weight are administered together 2 times a day for a week.
With body weight over 40 kg:
- combination with two antibiotics. Nexium 20 mg, amoxicillin 1 g and clarithromycin 500 mg are administered together 2 times a day for a week.
Children over 1 year and under 12 years
can take Nexium in another dosage form.
The tablets should be swallowed whole with liquid. Tablets should not be chewed or crushed. For patients with difficulty swallowing, you can dissolve the tablet in half a glass of still water (do not use other liquids, since the protective shell of the microgranules may dissolve), stir until the tablet disintegrates and drink the suspension of microgranules immediately or within 30 minutes. Then you should fill the glass halfway with water again, stir the rest and drink. Microgranules should not be chewed or crushed.
For patients who are unable to swallow, the tablets should be dissolved in still water and administered through a nasogastric tube. It is important that the syringe and probe chosen are thoroughly tested.
Administration of the drug through a nasogastric tube
1. Place the tablet in the syringe and fill the syringe with 25 ml of water and approximately 5 ml of air. For some probes, it may be necessary to dilute the drug in 50 ml of drinking water in order to prevent clogging of the probe with tablet granules.
2. Immediately shake the syringe for approximately 2 minutes to dissolve the tablet.
3. Hold the syringe with the tip facing up and make sure that the tip is not clogged.
4. Insert the tip of the syringe into the probe, continuing to keep it pointed upward.
5. Shake the syringe and turn it upside down. Immediately inject 5-10 ml of the dissolved drug into the tube. After injection, return the syringe to its original position and shake (the syringe should be held with the tip up to avoid clogging of the tip).
6. Turn the syringe over with the tip down and inject another 5-10 ml of the drug into the probe. Repeat this operation until the syringe is empty.
7. If part of the drug remains in the form of sediment in the syringe, fill the syringe with 25 ml of water and 5 ml of air and repeat the operations described in point 5. For some probes, 50 ml of drinking water may be needed for this purpose.
What does the drug treat?
Nexium tablets and other medications are used to treat various pathologies of the digestive system. In particular, the drug is considered effective for stomach ulcers caused by the bacterium Helicobacter pylori. Gastroesophageal reflux disease can also be successfully treated with this medicine. Thanks to him, you can quickly:
- Stabilize the condition.
- Prevent relapses.
- Remove negative symptoms.
As a prophylactic agent, Nexium, the instructions indicate this, is effective against the background of long-term treatment with non-steroidal anti-inflammatory drugs.
It also improves the condition of patients when diagnosing Zollinger-Ellison syndrome or other pathologies characterized by hypersecretion of the glands of the digestive organ.
A contraindication to taking the drug is intolerance to the active substance or other components that are included in the composition. The medicine is not prescribed for children under 12 years of age. They are prescribed treatment with caution for problems with kidney function. Also, when prescribing, be sure to take into account interactions with other medications.
Nexium
Nexium ®
(lat.
Nexium ®
) is an antiulcer drug, proton pump inhibitor (PPI).
Composition of Nexium
Nexium is available as extended-release capsules (enteric-coated pellets), 10 mg, film-coated tablets of 20 mg and 40 mg, and as a lyophilisate for solution for IV administration. The active substance of Nexium is esomeprazole. One capsule of Nexium extended-release contains:
- esomeprazole magnesium trihydrate 11.1 mg (equivalent to 10 mg esomeprazole)
- excipients: copolymer of methacrylic acid and ethyl acrylate, talc, sucrose in the form of spherical granules ranging in size from 0.25 to 0.355 mm, hyprolose, hypromellose, triethyl citrate, magnesium stearate, glycerol monostearate, polysorbate, dextrose, crospovidone, xanthan gum, citric acid, dye iron oxide yellow
One Nexium tablet contains:
- active substances: 22.3 mg or 44.5 mg of esomeprazole magnesium trihydrate, corresponding to 20 mg and 40 mg of esomeprazole
- excipients: glyceryl monostearate 40-55, hyprolose, hypromellose, red iron oxide, yellow iron oxide (for a dosage of 20 mg), magnesium stearate, methacrylic and ethacrylic acid copolymer (1:1), microcrystalline cellulose, synthetic paraffin, macrogol, polysorbate 80, crospovidone, sodium stearyl fumarate, spherical sucrose granules, titanium dioxide, talc, triethyl citrate.
One bottle of Nexium lyophilisate contains 42.5 mg of esomeprazole sodium, which corresponds to the content of 40 mg of esomeprazole.
Indications for use of Nexium
Gastroesophageal reflux disease (GERD):
- treatment of erosive reflux esophagitis
- long-term maintenance treatment after healing of erosive reflux esophagitis to prevent relapse
- symptomatic treatment of GERD
Peptic ulcer of the stomach and duodenum: As part of combination therapy (monotherapy with Nexium is not used for HP eradication):
- treatment of duodenal ulcer associated with Helicobacter pylori (Hp)
- prevention of relapses of peptic ulcer disease associated with Hp
With long-term use of non-steroidal anti-inflammatory drugs (NSAIDs):
- healing of stomach ulcers associated with taking NSAIDs
- prevention of gastric and duodenal ulcers associated with taking NSAIDs in patients at risk
Zollinger-Ellison syndrome
or other conditions characterized by pathological hypersecretion, including idiopathic hypersecretion.
Method of administration of Nexium and dose
The Nexium tablet is swallowed whole with liquid.
The tablets are not chewed or crushed. If you have problems swallowing, dissolve the tablet until the tablet disintegrates into microgranules in 100 ml of still water, after which all microgranules are drunk immediately or within half an hour, then add another 100 ml of still water, stir the remainder and drink. If oral therapy is not possible, a Nexium solution prepared from lyophilisate is administered intravenously based on a dose of 20-40 mg of esomeprazole once a day.
GERD (children over 12 years old and adults)
- one or two courses (the second - if the first is not enough to heal or get rid of the symptoms of esophagitis), each for 40 days, 40 mg of Nexium once a day
- maintenance therapy after healing of esophagitis - 20 mg Neximum once a day for a long period
- symptomatic treatment of GERD without esophagitis - 20 mg Nexium once a day; symptoms should disappear within 4 weeks
Stomach and duodenal ulcers (adults only)
- treatment of DU or prevention of PU associated with HP - Nexium 20 mg, amoxicillin 1 g and clarithromycin 500 mg twice a day for a week; it is possible to use Nexium as part of other eradication regimens ( see “Standards for diagnosis and treatment of acid-dependent and Helicobacter pylori-associated diseases (4th Moscow agreement)”)
. - treatment of ulcer associated with NSAID use - a maximum of 20 mg or 40 mg once a day for 4-8 weeks
- prevention of peptic ulcer associated with NSAID use - Nexium 20 mg or 40 mg once a day
Conditions associated with pathological hypersecretion (adults only)
, including Zollinger-Ellison syndrome and idiopathic hypersecretion. The starting dose is 40 mg Nexium twice daily. Next, the dose is selected individually, the duration of treatment depends on the development of the disease.
Comparison of Nexium with other acid-blocking drugs
It is generally accepted that proton pump inhibitors (PPIs) are the most effective class of drugs among antisecretory drugs that block the secretion of hydrochloric acid in the stomach. Nexium is the most modern PPI drug. The patent protection period for esomeprazole has not expired and there are no generics or other drugs with the active substance esomeprazole on the pharmaceutical market. However, among gastroenterologists there are different opinions regarding the exclusivity of Nexium among other drugs used to treat acid-related diseases. Recognizing its advantages in some of the parameters, especially over omeprazole, lansoprazole and pantoprazole and H2-blockers, a number of gastroenterologists (not all) believe that Nexium is inferior to Pariet (rabeprazole). This issue is discussed in more detail in the section Comparison of esomeprazole with other proton pump inhibitors
of the article
Esomeprazole
. It is important to note that the cost of Nexium and Pariet significantly exceeds the cost of other PPIs, and the effectiveness, even according to its “apologists,” is not very significant. In addition, the reaction of an individual organism to any antisecretory agent is strictly individual and the “best” medicine does not always cure a particular person. To select a medication and an individual dose, intragastric pH-metry is used, which is used to determine the patient’s response to the drug (see S.I. Rapoport et al. Selection of individual drug therapy for various diseases).
Preparation of Nexium solutions for injections and infusions from lyophilisate and their use
When preparing a Nexium solution for intravenous injection, 5 ml of a 0.9% sodium chloride solution for intravenous administration is added to the vial with Nexium lyophysate.
When preparing an infusion solution of Nexium, the contents of one bottle of Nexium lyophilisate are dissolved in 100 ml of 0.9% sodium chloride solution for intravenous administration. Nexium solution is a clear, pale yellow liquid. The degradation of the solution depends mainly on its acidity, so only 0.9% sodium chloride solution for intravenous administration should be used. Nexium solution should not be mixed or administered with other medications. The solution should not contain visible mechanical impurities or discoloration. The solution must be transparent. When prescribing 20 mg of Nexium per day, half of the prepared solution is administered, the rest is destroyed.
Nexium solution for injection is administered immediately after preparation intravenously for at least 3 minutes. Nexium solution for infusion is administered over 10–30 minutes.
Pharmacological properties of Nexium
The pharmacokinetics and pharmacodynamics of Nexium are determined by the active substance and are described in the article esomeprazole in the sections pharmacokinetics of esomeprazole
and
pharmacodynamics of esomeprazole
.
Contraindications for taking Nexium
- hypersensitivity to esomeprazole, substituted benzimidazoles or other ingredients included in the drug
- hereditary fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase deficiency
- age up to 12 years (for GERD) and any child age according to indications other than GERD
- Nexium should not be taken with atazanavir
- with caution in severe renal failure
Use of Nexium during pregnancy and breastfeeding
During pregnancy, Nexium therapy is possible only for health reasons. While taking Nexium, you must stop breastfeeding.
Side effects of Nexium
Headache, abdominal pain, diarrhea, flatulence, nausea, vomiting, constipation, dermatitis, itching, urticaria, rash, dizziness, dry mouth, insomnia, paresthesia, drowsiness, increased activity of liver enzymes, peripheral edema.
Long-term or high-dose use of Nexium increases the risk of hip, wrist, and spine fractures (“FDA Warns”).
Nexium overdose
To date, extremely rare cases of intentional overdose of Nexium have been described. Taking Nexium at a dose of 280 mg was accompanied by general weakness and gastrointestinal symptoms. A single dose of 80 mg of Nexium does not cause any negative effects. Specific antidotes are unknown. Esomeprazole binds to plasma proteins, making dialysis ineffective. In case of overdose, symptomatic and general supportive treatment is provided.
Special instructions for Nexium therapy
If any alarming symptoms are present (eg, significant spontaneous weight loss, repeated vomiting, dysphagia, hematemesis, or melena), or if a gastric ulcer is present or suspected, the presence of a malignant neoplasm must be excluded, since treatment with Nexium may lead to flattening symptoms and delay diagnosis.
Those taking Nexium for a long period (especially more than a year) should be under regular medical supervision. Those taking Nexium "as needed" should be instructed to contact their doctor if their symptoms change. Since there are fluctuations in the concentration of esomeprazole in plasma when prescribing therapy “as needed”, it is necessary to take into account the interaction of the drug with other drugs. Clarithromycin is a potent inhibitor of CYP3A4, therefore, when prescribing eradication therapy to patients receiving other drugs metabolized by CYP3A4 (for example, cisapride), possible contraindications and interactions of clarithromycin with these drugs must be taken into account. Nexium contains sucrose and is therefore contraindicated in patients with hereditary fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase deficiency.
Interaction of Nexium with other drugs
The interaction of Nexium with other drugs is determined by its active substance and is described in the article esomeprazole, in the section interaction of esomeprazole with other drugs
.
Instructions for medical use of Nexium
Official instructions for medical use of the drug Nexium from the manufacturer, pdf:
- Nexium, film-coated tablets containing 20 or 40 mg esomeprazole
- Nexium, enteric-coated pellets and granules for oral suspension
- Nexium, lyophilisate for the preparation of solution for intravenous administration
- Instructions (medical guide) for US patients “Nexium - capsules and extended-release suspension” (in English): “Medication Guide Nexium (esomeprazole magnesium) Delayed-Release Capsules, Nexium (esomeprazole magnesium) for Delayed-Release Oral Suspension.”
Nexium does not affect the ability to drive a car
or operate machinery.
According to the pharmacological index, Nexium belongs to the group “Proton pump inhibitors”«
. According to ATC, it belongs to the group “Proton pump inhibitors” and has the code A02BC05.
Medicines containing the active ingredient esomeprazole
Manufacturer of Nexium: AstraZeneca AB (AstraZeneca), Sweden.
In addition to Nexium, the following drugs with the active substance esomeprazole are registered in Russia: Neo-Zext, Esomeprazole-Vial, Esomeprazole Zentiva, Esomeprazole Canon, Esomeprazole-native, Emanera, Emezol.
In some other countries, brands are registered and produced: Nexium and Nexium IV (USA), Esopral (Italy, the Netherlands), Axagon (Italy), Nexiam (Belgium, Luxembourg, South Africa), as well as generics of esomeprazole: Ezocar (Belarus, Palestine), Sompraz (India, Myanmar), Neksium and Neksium Inj (India), Nexpro (India), etc.
Materials for healthcare professionals regarding the treatment of gastrointestinal diseases with Nexium
Articles
- Ivashkin V.T., Nemytin Yu.V., Makarov Yu.S. et al. Comparative assessment of the antisecretory activity of Losec MAPS, Pariet and Nexium in patients with peptic ulcer. TsVKG im. A.A. Vishnevsky, MMA named after. THEM. Sechenov.
- Zvyagin A.A., Shcherbakov P.L., Pochivalov A.V., Kashnikov V.V. Esomeprazole (Nexium) in the treatment of functional dyspepsia in children according to 24-hour pH monitoring // Bulletin of Siberian Medicine. — Appendix 2. — 2005.
- Demyanenko D. Nexium (esomeprazole) is a new word in the treatment of acid-related diseases of the gastrointestinal tract // Medical newspaper “Health of Ukraine”. – 2008. – No. 6/1. - With. 36–37.
- Grinevich V.B., Uspensky Yu.P. Secretolytic therapy of acid-dependent diseases of the digestive system from the position of a clinician: 2003 // Experimental and clinical gastroenterology. – 2003. – No. 6.
- Rudakova A.V. Pharmacoeconomic aspects of the use of rabeprazole and esomeprazole in patients with gastroesophageal reflux disease // Consilium-Medicum. – 2006. – Volume 8. – No. 2.
- Dobrovolsky O.V., Serebrova S.Yu. Endoscopic pH-metry as a method for assessing the effect of Nexium on the acid-producing function of the stomach in hyperacid gastritis // Evidence-based medicine - the basis of modern healthcare: abstracts of the VI International Congress. - Khabarovsk. – 2007. – P. 107-109.
- Bordin D.S., Berezina O.I., Yanova O.B., Kim V.A. The effectiveness of esomeprazole in the treatment of gastroesophageal reflux disease // Gastroenterology. 2015. No. 2. pp. 20–25.
- Sarsenbaeva A.S., Petukhova T.P. et al. Efficacy of standard first-line triple eradication therapy based on esomeprazole and other PPIs in patients with H. pylori-associated gastritis // Breast Cancer. Gastroenterology. – 2021. – No. 17. P. 1215-1219.
On the website GastroScan.ru in the “Literature” section there is a subsection “Esomeprazole”, containing articles for doctors regarding the treatment of diseases of the gastrointestinal tract with Nexium.
Video
Still from video: Popova E.N. Gastroesophageal reflux disease
Still from video Osadchuk A.M. Gastroesophageal reflux disease: current issues of diagnosis and treatment
Still from video Bordin D.S. A simple and effective algorithm for the treatment of acid-related diseases
Still from a video for pediatricians Mukhametova E.M.
Ipatova M.G. Symptoms of gastroesophageal reflux in a child: when is additional examination needed? On the website in the “Video” section there is a subsection for patients “Popular Gastroenterology” and a subsection “For Doctors”, containing video recordings of reports, lectures, webinars in various areas of gastroenterology for healthcare professionals.
Nexium has contraindications, side effects and application features; consultation with a specialist is necessary. Back to section
Negative effects and overdose
The active substance is well tolerated. Therefore, negative reactions of the body rarely occur when using the drug for the prevention and treatment of various diseases. The following symptoms indicate drug intolerance:
- Dry mouth.
- Increased body temperature.
- Bronchial spasm.
- Swelling.
- Hyperhidrosis.
Sometimes Nexium can provoke disturbances in the functioning of the central nervous system: depression, drowsiness, dizziness. In severe cases, increased excitability and hallucinations may occur.
There are certain risks of disruptions in the gastrointestinal tract, disorders of the liver and problems with hematopoietic processes.
In case of an overdose, which can be caused by an amount of active substance exceeding 280 mg, severe weakness and negative effects on the digestive system occur. There is no specific antidote. Therefore, all that is needed is to refuse treatment with the drug and carry out symptomatic treatment. Dialysis is ineffective.
Rules of application
Nexium 20 mg tablets, the instructions focus on this, you need to swallow. Be sure to drink them with enough water. If you have problems swallowing, you can dissolve the tablet in 100 g of still water to obtain microgranules. You need to drink the solution, and after half an hour you should fill the glass halfway with water, stir the rest and drink. Alternatively, the medicinal solution can be administered using a probe.
If it is impossible to take the medicine orally, use lyophilisate. A solution is prepared from it for intravenous administration. The recommended dose is 20-40 mg, frequency of administration is once a day.
Always during treatment, the dosage and duration of treatment are determined by the doctor based on the severity of the disease and the patient’s condition. At the same time, it is necessary to monitor the results obtained during the treatment process.
Nexium®
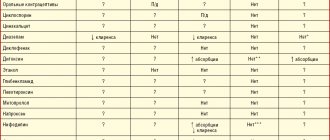
The effect of esomeprazole on the pharmacokinetics of other drugs. A decrease in the secretion of hydrochloric acid in the stomach during treatment with esomeprazole and other proton pump inhibitors can lead to a decrease or increase in the absorption of drugs, the absorption of which depends on the acidity of the environment. Like other drugs that reduce gastric acidity, treatment with esomeprazole may result in decreased absorption of ketoconazole, itraconazole and erlotinib, as well as increased absorption of drugs such as digoxin. Co-administration of omeprazole 20 mg once daily and digoxin increases the bioavailability of digoxin by 10% (the bioavailability of digoxin increased by up to 30% in 20% of patients).
Omeprazole has been shown to interact with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. An increase in pH during omeprazole therapy may affect the absorption of antiretroviral drugs. Interaction at the level of the CYP2C19 isoenzyme is also possible. When omeprazole is co-administered with certain antiretroviral drugs, such as atazanavir and nelfinavir, during omeprazole therapy, a decrease in their serum concentrations is observed. Therefore, their simultaneous use is not recommended. Co-administration of omeprazole (40 mg once daily) with atazanavir 300 mg/ritonavir 100 mg in healthy volunteers resulted in a significant decrease in the bioavailability of atazanavir (area under the conpo-required curve). When co-administered 30 mg of esomeprazole and diazepam, which is a substrate of the CYP2C19 isoenzyme , there is a decrease in diazepam clearance by 45%.
The use of esomeprazole at a dose of 40 mg led to an increase in residual phenytoin concentrations in patients with epilepsy by 13%. In this regard, it is recommended to monitor plasma concentrations of phenytoin when starting treatment with esomeprazole and when discontinuing it.
The use of omeprazole at a dose of 40 mg once a day led to an increase in the area under the curve “conSpecial instructions”).
With the simultaneous use of esomeprazole and tacrolimus, an increase in the concentration of tacrolimus in the blood serum was noted.
In some patients, an increase in the concentration of methotrexate was observed during combined use with proton pump inhibitors. When using high doses of methotrexate, the possibility of temporary withdrawal of esomeprazole should be considered.
Nexium® does not cause clinically significant changes in the pharmacokinetics of amoxicillin and quinidine.
Studies evaluating short-term co-administration of esomeprazole and naproxen or rofecoxib did not reveal a clinically significant pharmacokinetic interaction.
Effect of drugs on the pharmacokinetics of esomeprazole.
The isoenzymes CYP2C19 and CYP3A4 are involved in the metabolism of esomeprazole. The combined use of esomeprazole with clarithromycin (500 mg 2 times a day), which inhibits the CYP3A4 isoenzyme, leads to an increase in the AUC value of esomeprazole by 2 times. Co-administration of esomeprazole and a combined inhibitor of CYP3A4 and CYP2C19 isoenzymes, for example, voriconazole, may lead to a more than 2-fold increase in the AUC value for esomeprazole. As a rule, in such cases no dose adjustment of esomeprazole is required. Dose adjustment of esomeprazole may be required in patients with severe liver dysfunction and with long-term use. Long-term use of the drug is not indicated for children and adolescents under 12 years of age. Drugs that induce CYP2C19 and CYP3A4 isoenzymes, such as rifampicin and St. John's wort preparations, when used together with esomeprazole, may lead to a decrease in the concentration of esomeprazole in the blood plasma by accelerating the metabolism of esomeprazole
Treatment during pregnancy and lactation
No studies have been conducted on the effect of the drug on the fetus during pregnancy. Therefore, it is allowed to prescribe the drug for the treatment of various pathologies during pregnancy in cases where there are guarantees that the benefits to the woman’s body are significantly higher than the possible risks to the fetus.
There is no data on the passage of the active substance into breast milk, therefore, to minimize the risks to the newborn baby during the treatment period, it is recommended to stop breastfeeding.
Nexium, 28 pcs., 40 mg, film-coated tablets
The effect of esomeprazole on the pharmacokinetics of other drugs. A decrease in the secretion of hydrochloric acid in the stomach during treatment with esomeprazole and other proton pump inhibitors can lead to a decrease or increase in the absorption of drugs, the absorption of which depends on the acidity of the environment. Like other drugs that reduce gastric acidity, treatment with esomeprazole may result in decreased absorption of ketoconazole, itraconazole and erlotinib and increased absorption of drugs such as digoxin. Co-administration of omeprazole 20 mg once daily and digoxin increases the bioavailability of digoxin by 10% (the bioavailability of digoxin increased by up to 30% in 2 out of 10 patients).
Omeprazole has been shown to interact with some antiretroviral drugs. The mechanisms and clinical significance of these interactions are not always known. An increase in pH during omeprazole therapy may affect the absorption of antiretroviral drugs. Interaction at the level of the CYP2C19 isoenzyme is also possible. When omeprazole is co-administered with certain antiretroviral drugs, such as atazanavir and nelfinavir, a decrease in their serum concentrations is observed during omeprazole therapy. Therefore, their simultaneous use is not recommended. Co-administration of omeprazole (40 mg once daily) with atazanavir 300 mg/ritonavir 100 mg in healthy volunteers resulted in a significant decrease in the bioavailability of atazanavir (AUC, Cmax and Cmin decreased by approximately 75%). Increasing the atazanavir dose to 400 mg did not compensate for the effect of omeprazole on the bioavailability of atazanavir.
With the simultaneous use of omeprazole and saquinavir, an increase in the concentration of saquinavir in the serum was noted; when used with some other antiretroviral drugs, their concentration did not change. Given the similar pharmacokinetic and pharmacodynamic properties of omeprazole and esomeprazole, co-administration of esomeprazole with antiretroviral drugs such as atazanavir and nelfinavir is not recommended.
Esomeprazole inhibits CYP2C19, the main isoenzyme involved in its metabolism. Accordingly, the combined use of esomeprazole with other drugs in the metabolism of which the CYP2C19 isoenzyme is involved, such as diazepam, citalopram, imipramine, clomipramine, phenytoin, etc., may lead to increased plasma concentrations of these drugs, which in turn may require a dose reduction . This interaction is especially important to remember when using Nexium® in an as-needed regimen.
When 30 mg of esomeprazole and diazepam, which is a substrate of the CYP2C19 isoenzyme, are taken together, a decrease in the clearance of diazepam by 45% is observed. The use of esomeprazole at a dose of 40 mg led to an increase in residual phenytoin concentrations in patients with epilepsy by 13%. In this regard, it is recommended to monitor plasma concentrations of phenytoin when starting treatment with esomeprazole and when discontinuing it.
The use of omeprazole at a dose of 40 mg 1 time per day led to an increase in the AUC and Cmax of voriconazole (CYP2C19 isoenzyme substrate) by 15 and 41%, respectively.
Co-administration of warfarin with 40 mg esomeprazole does not lead to a change in coagulation time in patients taking warfarin for a long time. However, several cases of clinically significant increases in the INR index have been reported with the combined use of warfarin and esomeprazole. It is recommended to monitor the INR at the beginning and at the end of the combined use of esomeprazole and warfarin or other coumarin derivatives.
According to the results of the studies, a pharmacokinetic/pharmacodynamic interaction was noted between clopidogrel (loading dose - 300 mg and maintenance dose - 75 mg / day) and esomeprazole (40 mg / day, orally), which leads to a decrease in exposure to the active metabolite of clopidogrel by an average of 40% and maximum inhibition of ADP-induced platelet aggregation by an average of 14%.
The clinical significance of this interaction is unclear. In a prospective study of patients receiving placebo or omeprazole at a dose of 20 mg/day concomitantly with therapy with clopidogrel and acetylsalicylic acid (ASA), and in an analysis of the clinical outcomes of large-scale randomized trials, there was no increase in the risk of cardiovascular complications with the combined use of clopidogrel and inhibitors proton pump, including esomeprazole.
The results of a number of observational studies are contradictory and do not provide a clear answer about the presence or absence of an increased risk of thromboembolic cardiovascular complications during the combined use of clopidogrel and proton pump inhibitors.
When clopidogrel was used together with a fixed combination of 20 mg esomeprazole and 81 mg ASA, exposure to the active metabolite of clopidogrel decreased by almost 40% compared with clopidogrel monotherapy, while the maximum levels of inhibition of ADP-induced platelet aggregation were the same, which is likely due to simultaneous administration ASA in low dose.
The use of omeprazole at a dose of 40 mg led to an increase in Cmax and AUC of cilostazol by 18 and 26%, respectively; for one of the active metabolites of cilostazol, the increase was 29 and 69%, respectively.
Co-administration of cisapride with 40 mg of esomeprazole leads to an increase in the values of the pharmacokinetic parameters of cisapride in healthy volunteers: AUC by 32% and T1/2 by 31%, however, the Cmax of cisapride in plasma does not change significantly. The slight prolongation of the QT interval, which was observed with cisapride monotherapy, did not increase with the addition of Nexium® (see "Special Instructions").
With the simultaneous use of esomeprazole and tacrolimus, an increase in the concentration of tacrolimus in the blood serum was noted.
In some patients, an increase in the concentration of methotrexate was observed during combined use with proton pump inhibitors. When using high doses of methotrexate, the possibility of temporary withdrawal of esomeprazole should be considered.
Nexium® does not cause clinically significant changes in the pharmacokinetics of amoxicillin and quinidine.
Studies evaluating short-term co-administration of esomeprazole and naproxen or rofecoxib did not reveal a clinically significant pharmacokinetic interaction.
Effect of drugs on the pharmacokinetics of esomeprazole. The isoenzymes CYP2C19 and CYP3A4 are involved in the metabolism of esomeprazole. The combined use of esomeprazole with clarithromycin (500 mg 2 times a day), which inhibits the CYP3A4 isoenzyme, leads to an increase in the AUC value of esomeprazole by 2 times.
Co-administration of esomeprazole and a combined inhibitor of CYP3A4 and CYP2C19 isoenzymes, such as voriconazole, may result in a more than 2-fold increase in the AUC value for esomeprazole. As a rule, in such cases no dose adjustment of esomeprazole is required. Dose adjustment of esomeprazole may be required in patients with severe liver dysfunction and with long-term use.
Drugs that induce CYP2C19 and CYP3A4 isoenzymes, such as rifampicin and St. John's wort preparations, when used together with esomeprazole, may lead to a decrease in the concentration of esomeprazole in the blood plasma by accelerating the metabolism of esomeprazole.
special instructions
Side effects when taking Nexium may mask symptoms of the development of cancer pathologies. Therefore, if vomiting and weight loss occur, you should definitely undergo additional examination.
With long-term use, it is necessary to ensure control over the patient's condition. If there are any negative reactions of the body, treatment is stopped. The doctor may prescribe a medicine with a different composition and similar effects.
The drug Nexium does not affect psychomotor reactions, therefore it has no restrictions when driving a car. Drinking alcohol significantly reduces the effectiveness of the drug.