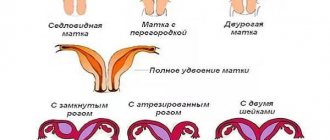
When trying unsuccessfully to get pregnant, women begin to pay more attention to the menstrual cycle. They calculate the “stray” days and carefully monitor the duration of menstruation, forgetting to check the quality of ovulation. But the presence of critical days is not yet an indicator that the body is ovulating. The anovulatory cycle (anovulation) is one of the most common causes of female infertility.
Appointment with a gynecologist - 1000 rubles. Comprehensive pelvic ultrasound - 1000 rubles. Appointment based on ultrasound or test results - 500 rubles (optional)
CLICK TO MAKE AN APPOINTMENT, TEST OR ULTRASOUND
What is ovulation and how is it related to pregnancy?
The content of the article
The body of a healthy sexually mature woman is fully adapted for conception, bearing a child and childbirth. The fact that everything is in order with the reproductive system can be judged by the stability of monthly processes - ovulation and menstruation.
Ovulation is the maturation and release of a mature egg from the ovary into the fallopian tubes for fertilization. Menstruation is bleeding that removes from the uterus the mucous layer (endometrium), which forms each new cycle, and the unfertilized egg. If fertilization occurs, the egg attaches to the walls of the uterus, and menstruation does not begin.
After childbirth, the cycle resumes, and so on every month from year to year. But sometimes, for some reason, the egg is not formed or does not mature. A pathological condition in which the release of an egg ready for fertilization does not occur is called anovulation.
The anovulatory cycle is a period of menstrual bleeding in one phase, during which there is no ovulation and formation of the corpus luteum. The regularity of menstruation may be maintained.
There are two types of anovulatory cycle:
- physiological - normal;
- pathological - requiring treatment.
Anovulation can occur from time to time, up to several times a year, and this is considered normal. With age, such failures occur more often, smoothly flowing into menopause. But it happens that even a young woman does not ovulate at all - this is a good reason for diagnosing and treating infertility.
Types and causes of endocrine infertility
A woman's reproductive health is regulated by the endocrine interaction between the pituitary gland, hypothalamus and ovaries. The key hormonal factors in this regard are luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as estradiol. Anovulatory infertility can be caused by any disorder in this chain:
- Insufficiency of gonadotropic hormones (type I);
- Polycystic ovary syndrome (type II);
- Premature depletion of ovulatory reserve (type III).
Less common factors that provoke anovulation include hypothyroidism and hyperprolactinemia. Diagnosis and therapy for each type of disease has its own characteristics.
What is an anovulatory cycle?
As the name suggests, an anovulatory cycle is a period when a woman misses ovulation. For those who do not plan to have children, use contraception, or do not attach any importance to it, anovulation often occurs unnoticed. This is due to the peculiarity of menstrual bleeding - periods can occur as usual. In a normal menstrual cycle, the production of the hormone progesterone, which regulates bleeding during menstrual periods, is stimulated by the release of the egg. It is this hormone that helps a woman’s body maintain regular periods. During the anovulatory cycle, insufficient levels of progesterone can cause uterine bleeding of a different nature, which is mistaken for menstruation.
Should you see a doctor?
As a rule, a woman begins to track her ovulation only when she is planning a pregnancy. The anovulatory cycle is the most common cause of infertility. Therefore, it is imperative to seek qualified medical help from a gynecologist.
Only a doctor can determine the exact cause of the lack of ovulation and develop the optimal treatment regimen to restore sexual function. The gynecologist has all the necessary equipment to fully diagnose the pathological condition. You should not neglect routine examinations with a doctor, because it is quite difficult to suspect such a problem on your own.
The volume of blood lost and the regularity of menstruation during an anovulatory cycle can be the same as during full menstruation. Regular examinations will allow you to identify pathology at an early stage and avoid serious consequences.
Causes of anovulation
A menstrual cycle without ovulation is most common in two different age groups:
- Girls who have just entered the period of growing up
. Teens typically experience anovulatory cycles for a full year after their first period (menarche). - Women approaching menopause (age 40 to 50 years)
. Significant hormonal changes occur in their body.
In both cases, the lack of ovulation is caused by sudden changes in hormone levels.
Women of the active childbearing period have chronic anovulation. Causes:
- hormonal (endocrine) disorders, ovarian dysfunction;
- inflammatory diseases of the reproductive system, STIs;
- too high (obesity) or too low body weight (anorexia);
- extreme physical activity;
- late puberty, early menopause;
- high levels of the hormone prolactin;
- severe stress
- climate change.
There are other possible reasons, for example, underdevelopment of the ovaries, genetic problems, etc.
Indirect signs of anovulation, indicating hormonal disorders:
- hair growth on the face and body in uncharacteristic places;
- acne;
- bleeding or spotting outside the cycle;
- problems with conception.
Menstrual cycle in the late reproductive period: disorders and reasonable correction
The article discusses strategies for the prevention and treatment of menstrual dysfunction in the late reproductive period. Particular attention is paid to the possibilities of hormonal therapy during the menopausal transition. Data from observational studies demonstrate the benefit of combined oral contraceptives for painful menstruation and abnormal uterine bleeding. The use of combined oral contraceptives to regulate the menstrual cycle is also a method of preventing proliferative endometrial diseases.
The late reproductive period is one of those periods of a woman’s life when the restructuring of homeostatic mechanisms against the background of a changing hormonal background requires the coordinated work of adaptation systems. Adaptation disorders at this stage of the life cycle more often than ever are manifested by disorders of menstrual function and numerous psycho-vegetative symptoms [1]. It must be understood that the parameters of a normal menstrual cycle for young women are not acceptable for women in the late reproductive period, which is characterized by a different “norm/pathology” ratio. Underestimation of age-related features of the functioning of the reproductive system can lead to both overdiagnosis and unjustified therapeutic interventions, and to untimely detection of latent gynecological pathology. This determines the relevance of adequate monitoring of women in the late reproductive period.
A woman enters the late reproductive period of life after 35 years. Since the age of 40, the state of her reproductive system is often referred to as “perimenopause.” If the late reproductive period and premenopause are determined by age, then the menopausal transition and perimenopause reflect the functional characteristics of the state of the reproductive system of women. The concepts of “menopausal transition” and “perimenopause” are not identical, but very close. The menopausal transition begins when menstrual cycles lose stable duration due to an isolated increase in follicle-stimulating hormone (FSH) levels, and ends with the last menstrual period. Perimenopause begins at the same time as the menopausal transition, but extends an additional 12 months after the last menstrual period. However, it is possible to determine the time of menopause (last menstruation) only after 12 months, so the one-year difference in the concepts of “perimenopause” and “menopausal transition” has a purely terminological meaning.
According to the modified classification of menopausal stages PENN-5 (a staging system in 5 groups to more accurately identify changes in cycle length), the menopausal transition has three stages, two of which occur during premenopause [2]. In the early phase of the menopausal transition, the menstrual cycle loses its regularity and becomes variable in the duration of intermenstrual intervals with deviations of 7 days or more. The late phase of the menopausal transition is characterized by the “missing” of two or more menstruation, the appearance of intermenstrual intervals lasting up to 60 days with an increase in FSH levels to 40 IU/l or more [3], and its onset allows one to predict the time of expected menopause in the range from 2.6 to 3.3 years [4]. The absence of menstruation for a year in a woman over 45 years of age in 90% of cases means the onset of menopause.
Contrary to early ideas about a linear decrease in ovarian secretion, a modern view of endocrine characteristics in women of the older reproductive period suggests significant fluctuations in hormone levels. The decline of ovarian function due to a decrease in the follicular pool, impaired folliculogenesis, increased apoptosis and atresia of the follicles begin long before perimenopause. The age-related decline in the number of follicles is biexponential and accelerates by more than 2 times when their number drops below 25 thousand (this occurs on average at 37.5 years). The time interval from the beginning of a decrease in the follicular pool to menopause is about 13 years [5]. Circulating FSH concentrations progressively increase during the late reproductive period and subsequent menopausal transition [6, 7], which is associated with a decrease in inhibin production rather than estradiol [7, 8]. The level of estradiol in perimenopause fluctuates along with the level of FSH, sometimes reaching values typical for women under 35 years of age, and decreasing significantly only towards the end of the menopausal transition. Progesterone levels, meanwhile, already in the early phase of the menopausal transition are lower than in middle reproductive age, despite monthly menstruation, and vary depending on body mass index, reflecting an inverse correlation with this anthropometric indicator [7]. In the late phase of the menopausal transition, along with a decrease in folliculogenesis, the frequency of anovulation increases, which is accompanied by a significant persistent decrease in progesterone secretion [8]. Testosterone levels do not change significantly during the menopausal transition [6].
Proteins produced by granulosa cells - inhibin and activin - also play an important role in the process of changes in hormonal homeostasis. Depending on the presence of one or another subunit, two heterodimers of inhibin are distinguished - inhibin A, a product of the corpus luteum, and inhibin B, secreted by the antral and dominant follicles. Accordingly, inhibin A levels increase during the luteal phase and inhibin B levels increase during the follicular phase, but both suppress pituitary FSH secretion. Activins, on the contrary, stimulate the pituitary release of FSH and are derivatives of inhibin A and inhibin B.
In the late reproductive period and throughout the menopausal transition, levels of inhibin B in the early follicular phase and inhibin A in the luteal phase decrease simultaneously with an increase in FSH concentrations [8]. The concentration of activin A in perimenopause, on the contrary, increases. Activin A affects FSH secretion to a lesser extent than inhibins, but the combined effect of these proteins on pituitary secretion provides an increase in FSH concentrations even in the absence of a decrease in ovarian estradiol synthesis.
Another marker of ovarian reserve, anti-Mullerian hormone (AMH), is secreted by granulosa cells of secondary and preantral follicles; its circulating concentration remains relatively stable during the menstrual cycle and correlates with the number of early antral follicles. AMH levels decrease significantly and progressively throughout the menopausal transition [9].
Thus, during perimenopause, the variety of endocrine changes determines the existence of numerous clinical variants of menopause, each of which can become the basis of a pathological condition and must be assessed from the standpoint of compliance with the physiological norm.
The diagnosis of menopausal transition is based on clinical signs. Measuring hormone concentrations does not identify the stage of the menopausal transition or accurately predict the timing of menopause. An isolated increase in FSH in the early follicular phase becomes evident in many women over 40 years of age and is associated with a prognosis of decreased fertility, but significant variation from cycle to cycle determines its low predictive value as a marker of cessation of menstruation [3]. Therefore, from a clinical point of view, a woman can be considered to have entered the menopausal transition when she begins to experience deviations in her regular menstrual cycle after the age of 40.
Diagnosis of these deviations is based primarily on the individual rhythm of menstruation formed during the reproductive period and, secondly, on ideas about the parameters of the normal menstrual cycle characteristic of mature reproductive age. In individual cases, it is allowed to vary these parameters within the following limits:
1. The duration of the menstrual cycle from the first day of menstruation to the first day of the next menstruation ranges from 21 to 42 days. Clinical diagnosis of the menopausal transition implies variability in the duration of the cycle, extending for a week or more, not beyond the specified limits, but beyond the limits of the stable cycle characteristic of a particular woman. A reduction in the menstrual cycle of less than 21 days is designated by the term “polymenorrhea”; a lengthening of more than 42 days is oligomenorrhea; absence of menstruation for 6 months or more is amenorrhea.
2. The number of days of menstrual bleeding is from 3 to 7 and the amount of blood lost is up to 80 ml. Menstrual bleeding refers to blood discharge, for the control of which during the day one standard hygiene product is not enough. Bloody discharge of lesser intensity is referred to as bleeding. The number of days of bleeding in a normal menstrual cycle ranges from 3 to 7, bleeding is not taken into account. Decreased duration of menstrual bleeding – hypomenorrhea; increase – menorrhagia. Menorrhagia also includes menstrual bleeding, accompanied by increased blood loss, which is assessed by the subjective feeling of increased menstrual flow or by the objective criterion of a decrease in hemoglobin levels after menstruation (you can also focus on the number of hygiene products used by a woman: if there is one hygiene product with the maximum ability to control bleeding lasts no more than 2 hours, it is legitimate to talk about menorrhagia).
3. Absence of bleeding and spotting, in addition to cyclic menstruation. Acyclic bleeding or spotting is referred to as metrorrhagia. Premenstrual and postmenstrual bleeding is considered pathological if the total number of days of menstruation exceeds a week. Acyclic bleeding can occur on any day of the menstrual cycle with a preserved menstrual rhythm, but can also represent a form of disturbance in the absence of a normal menstrual rhythm (for example, metrorrhagia - prolonged light bleeding/bruising after a delay in menstruation). In the presence of heavy acyclic bleeding, the term “menometrorrhagia” is used (the above example with heavy bleeding exceeding the normal menstrual flow characteristic of a woman). Menometrorrhagia is also understood as a combination of menorrhagia (heavy regular menstruation) and metrorrhagia (acyclic bleeding).
4. No complaints related to menstruation. A healthy woman should not feel either the approach of menstruation or any painful or other unpleasant symptoms during menstrual bleeding. Minor discomfort, however, is acceptable, but if premenstrual/menstrual symptoms disrupt the quality of life of a woman or her environment and force her to change her usual lifestyle, then it is necessary to consider them as a pathology, identify their causes (not always functional!) and resort to treatment. In the absence of an organic or extragenital substrate for disorders, complaints associated with the luteal phase of the menstrual cycle are classified as premenstrual syndrome, and pain during menstruation alone or in combination with autonomic disorders is classified as dysmenorrhea.
5. The end of menstrual function is no earlier than 40–45 years. Menopause usually occurs between the ages of 50 and 53. The cessation of menstruation before the age of 40 years is referred to as premature menopause, and in the interval from 40 to 45 years – early menopause.
In addition to menstrual irregularities, symptoms of perimenopause include the appearance of vasomotor and psychovegetative disorders. Classic examples of vasomotor disturbances—hot flashes and night sweats—are usually associated with a persistent decrease in estradiol secretion below the level of the early follicular phase of the normal menstrual cycle (
Deviations of the menstrual cycle associated with its variable duration are naturally the norm for perimenopause. But simultaneously with the formation of oligomenorrhea - amenorrhea in a woman, the nature of menstruation itself may change, acquiring the features of abnormal uterine bleeding. Abnormal uterine bleeding occurs as a result of relatively high acyclic estrogen production with relatively low progesterone production during the menopausal transition. But it is precisely this physiological endocrine feature of perimenopause that can cause an increased risk of developing endometrial hyperplasia and cancer. Among premenopausal women with abnormal uterine bleeding aged 45 years or more, there is a threefold increase in the risk of proliferative endometrial diseases. This is especially clearly seen in the dynamics of the prevalence of simple endometrial hyperplasia in different age groups [10]. Simple endometrial hyperplasia in perimenopause can be considered as a natural reaction of the mucous membrane of the uterine body to undisguised estrogen stimulation, that is, considered a variant of the norm if it is not accompanied by abnormal uterine bleeding.
Most often during the menopausal transition, abnormal uterine bleeding is represented by disorders that do not have an organic substrate and are called dysfunctional uterine bleeding. The cause of dysfunctional uterine bleeding remains unknown. Disturbances in ovulatory function of the ovaries are an inevitable part of the menopausal transition, but only a small proportion of anovulatory cycles end in bleeding. The identified relationship between the development of dysfunctional uterine bleeding and aggravated premorbid background, stressful situations, disorders in the reproductive system suggests that dysfunctional uterine bleeding is the result of a breakdown in the adaptive mechanisms of the reproductive system, which allows, under normal conditions, to “complete” the menstrual-like bleeding of the anovulatory cycle in normal terms and with normal blood loss.
Due to the real difficulties of separating the norm and pathology of the menstrual cycle in perimenopause, the strategy for the prevention and treatment of menstrual dysfunction in this period of a woman’s life consists of several components:
1. Symptoms that interfere with the quality of life of patients must be eliminated, namely: menorrhagia, menometrorrhagia, dysmenorrhea, manifestations of premenstrual syndrome, as well as various symptoms of hypothalamic and autonomic dysfunction.
2. Anovulation, characteristic of the menopausal transition, is associated with a high risk of hyperplastic processes in the reproductive system due to constant estrogen stimulation. Therefore, perimenopausal women should undergo regular examinations for the early detection of proliferative diseases and receive timely treatment and preventive care.
3. Menstrual dysfunction is often combined with various disorders, especially autonomic dysfunction and metabolic disorders, which should also be corrected even in the absence of complaints in order to avoid the formation of a pathological relationship between hormonal and metabolic abnormalities that support each other.
Monitoring women during the menopausal transition should be active, but not aggressive. Screening programs are adopted only in the practice of preventive measures to reduce the incidence of diseases of the cervix and mammary glands. There is no need to conduct any additional examination, including a gynecological examination, ultrasound examination of the pelvic organs, for a healthy woman during the menopausal transition, who has no complaints and is not at risk for endometrial cancer. Delays in menstruation, shortening of the menstrual cycle, episodes of oligomenorrhea and amenorrhea are not regarded as complaints or pathological symptoms. When forming risk groups for endometrial diseases, it should be remembered that the risk factors for atypical hyperplasia coincide with the risk factors for endometrial cancer [11]. In premenopause, these include obesity, type 2 diabetes mellitus, polycystic ovary syndrome, long-term menstrual irregularities during the reproductive period (chronic anovulation), absence of childbirth (provided that progestin-containing drugs are not taken). The structure of risk factors for simple hyperplasia is somewhat different. This fully fits into the concept put forward by A. Ferenczy and M. Gelfand and implying the presence of two “pathways for the endometrium”: the path of hyperplasia or neoplasia. This concept does not recognize the gradual increase in hyperplastic changes, but postulates cytological atypia as the only morphological discrete factor that distinguishes benign endometrial damage from potentially malignant processes.
Despite the relative rarity of detection of atypical hyperplasia and endometrial cancer in premenopause, menstrual irregularities in the form of abnormal uterine bleeding require examination due to oncological alertness. In case of polymenorrhea and menorrhagia, it is permissible to limit yourself to a gynecological examination and ultrasound on the 5–8th day from the onset of menstrual bleeding and plan further tactics for managing the woman depending on the results obtained. Metrorrhagia and menometrorrhagia deserve a more radical approach, since they often reflect severe endometrial pathology. Regardless of the results of ultrasound, patients with these forms of disorders are recommended to have a morphological examination of the endometrium obtained by biopsy or curettage.
Therapeutic and preventive measures aimed at controlling the menstrual cycle and preventing endometrial diseases should also not be carried out universally to all perimenopausal women. Healthy women who are not at risk for endometrial cancer, whose only complaint is an irregular cycle, can receive recommendations for a healthy lifestyle and explanations about the peculiarities of the menopause and symptoms that require medical intervention. As part of recommendations for maintaining a healthy lifestyle, the doctor can provide information about vitamin-mineral complexes and dietary supplements that can increase the body's adaptive capabilities and avoid the pathological course of menopause. The presence of abnormal uterine bleeding requires control of the menstrual cycle, taking into account the organic or functional substrate of bleeding and the woman’s additional goals.
For women who are sexually active, regardless of the frequency and regularity of sexual intercourse, the best option for regulating the menstrual cycle is hormonal contraception. It is fundamentally important that this type of medication can also be recommended for those women whose menopausal transition proceeds relatively smoothly, accompanied only by delayed menstruation. Control of the menstrual cycle can be effectively achieved using combined oral contraceptives (COCs), provided that the patient does not smoke and has no other contraindications to the use of this group of drugs. The use of COCs to prevent pregnancy is relevant until the end of perimenopause. A natural decrease in fertility after 37 years of age does not mean a decrease in the need for contraception, especially since pregnancy in women over 40 years of age is accompanied by an increased risk of congenital and chromosomal abnormalities of fetal development, spontaneous abortions, pregnancy complications, as well as an increase in maternal morbidity and mortality [12].
The choice of a contraceptive method in the age group over 40 years is dictated by many circumstances: the frequency of sexual intercourse, sexual problems, concomitant somatic diseases and the need for additional non-contraceptive effects, among which the first place is the regulation of the disrupted menstrual cycle [13]. According to Cochrane reviews, randomized controlled trials provide some evidence in favor of the effectiveness of COCs for the treatment of dysmenorrhea or reduction of menstrual blood loss [14, 15] compared with other types of therapy. Data from observational studies demonstrate a positive effect of COC use on painful menstruation and abnormal uterine bleeding [16, 17]. Based on these data, current clinical guidelines suggest the use of COCs as a treatment for severe uterine bleeding [18]. At the same time, the use of COCs to regulate the menstrual cycle is simultaneously becoming a method of preventing proliferative endometrial diseases. It is known that combined hormonal contraceptives reduce the risk of endometrial cancer [19, 20] in direct proportion to the duration of their use.
The benefits of regulating the disrupted menstrual cycle and preventing endometrial hyperplasia and cancer while achieving a contraceptive effect should be weighed against the possible risks of using COCs, which naturally increase with age.
If there are contraindications to the use of estrogen-containing contraceptives, control of abnormal uterine bleeding and endometrial hyperplasia in sexually active women can be achieved using progestogen-only contraceptives. Purely progestogen contraceptives include a large group of drugs, among them injectable, oral drugs, hormone-releasing intrauterine systems and implants. Continuous administration of progestogens in an adequate dose leads to decidualization of the stroma and atrophy of the endometrial glandular epithelium. The acceptability of a particular contraceptive is largely determined by the method of its administration.
The choice of a specific hormonal drug within these two groups is usually not regulated, although it is clear that different drugs have different potential both in regulating the menstrual cycle and in terms of safety of use. To date, two contraceptives are recognized as leaders in the menstrual cycle correction program in perimenopausal women: a combined oral contraceptive containing estradiol valerate and dienogest (Qlaira®), and a levonorgestrel-containing intrauterine system (LNG-IUS) (Mirena®) [21].
The combination of estradiol valerate and dienogest is registered in many countries as a treatment for menorrhagia [22]. The advantages of COC Claira® in the control of the menstrual cycle and excessive endometrial proliferation are the strong progestogenic potential of dienogest [23, 24], a dynamic four-phase dosing regimen that ensures estrogen dominance in the early phase of administration and progestin dominance in the middle and final phase of the cycle, as well as prolongation of administration until 26 days of a 28-day cycle. A comparison of Qlaira® with a low-dose COC containing levonorgestrel used in a standard regimen demonstrated a better profile of the Qlaira® contraceptive in terms of the number of bleeding days and blood loss over the monitored time intervals [25]. Placebo-controlled studies showed a significantly greater reduction in menstrual blood loss by 353–373 ml over a 90-day observation interval compared with placebo (130 ml), accompanied by a significant increase in hemoglobin levels and ferritin concentrations [26, 27].
During the menopausal transition period, when choosing a drug, it is extremely important to comply with the principle of safety. Age-related metabolic disorders, which begin to progressively increase in a woman as menopause approaches, should not be further provoked by medications. From a safety standpoint, Qlaira® has advantages over COCs containing ethinyl estradiol. Unlike ethinyl estradiol, which is resistant to hepatic metabolism, estradiol valerate is rapidly metabolized into hormones identical to natural estrogens: 17-beta-estradiol, estrone and estriol. The effect of 2-3 mg of 17-beta-estradiol on the synthesis of hepatic proteins is several times lower than the activity of 20-30 μg of ethinyl estradiol, which entails several times less pronounced activation of blood coagulation and the renin-angiotensin-aldosterone system and, therefore, , lower likelihood of adverse reactions and complications. At the same time, replenishment of the positive effects of endogenous hormones lost due to the suppression of folliculogenesis is achieved better than when using ethinyl estradiol-containing drugs. This is due to two circumstances. Firstly, taking Qlaira® compensates for the decrease in the production of all three estrogen fractions. Secondly, bioidentical metabolites of estradiol valerate exert their action not only through type 1 estrogen receptors (alpha type), like ethinyl estradiol, but also through type 2 receptors (beta type), which predominate in the blood vessels and central nervous system. Non-proliferative effects transmitted by type 2 receptors improve vascular function and have a positive effect on the exchange of neurotransmitters in the brain. As a result, the tolerability of the drug Qlaira® increases, which was noted in clinical studies [28].
It is possible that further research into the safety profile of contraceptives containing an analogue of natural estradiol will reduce the number of contraindications to their use compared to ethinyl estradiol-containing COCs. But while data are accumulating, when prescribing any combined hormonal contraceptives, clinicians should use the same eligibility criteria [29] and, if there are contraindications to the use of COCs, choose progestogen contraceptives for pregnancy protection and bleeding control.
Among progestogen contraceptives, the levonorgestrel-containing intrauterine system has a therapeutic indication for use in patients with heavy uterine bleeding in many countries [30]. According to the literature, the use of an LNG-IUD in women with menorrhagia leads to a reduction in menstrual blood loss by 80% within 3 months after insertion, and by 95% or more within 12 months [31]. A reduction in the volume and duration of menstrual bleeding with the use of the Mirena® LNG-IUS is accompanied by an increase in hemoglobin and ferritin levels. These properties of the LNG-IUS allow us to consider it as an alternative to medicinal oral and surgical treatment of menorrhagia [30, 31].
For women who are not sexually active, prevention of recurrent episodes of abnormal uterine bleeding is usually recommended with cyclic progestins. There is no consensus regarding the ability of progestins to control the menstrual cycle with a tendency to bleeding. Thus, the effectiveness of the oral form of medroxyprogesterone acetate, often prescribed in the USA in a cyclic regimen, has not been confirmed in clinical studies. Nevertheless, progestins remain one of the most popular methods for preventing endometrial hyperplasia and regulating the menstrual cycle [32] in perimenopause. Compliance with the principle of maximum safety of therapy involves the preferential administration of those progestins that have the most selective effect on the endometrium and minimal effect on metabolism.
Hormonal therapy does not exhaust all the possibilities of drug and non-drug therapy during the menopausal transition. Of course, in the process of monitoring patients, lifestyle modification and elimination of risk factors for diseases associated with menopause are necessary. However, hormonal therapy should not be neglected if there are gynecological indications for its use, since it is a powerful means of preventing many chronic diseases and helps improve the quality of life.
Signs that ovulation has not occurred
The main sign of anovulation is the absence of menstruation. The remaining symptoms are less noticeable, but they can be easily calculated if you know how the normal process proceeds.
Signs of normal ovulation, observed between 7-14 days after the last menstrual period:
- Changes in the nature and amount of vaginal discharge during the entire cycle - from liquid transparent to viscous, milky color.
- Soreness, tension of the mammary glands.
- Pulling sensations in the area of the ovaries, lower abdomen.
- Jumps in basal temperature.
- Increased libido.
More information about ways to determine ovulation is written in our article “What is ovulation.”
Why is there no ovulation?
Ovulation is the most important event of every menstrual cycle. This is the rupture of follicles (vesicles) on the ovary, during which a mature egg is released, necessary for fertilization. This does not happen with anovulation.
Anovulation is most often caused by a violation of the secretion of hormones: from the hormones of the hypothalamus through the hormones of the pituitary gland, up to the hormones of the ovaries, as a result of which there is no monthly maturation of the egg and, accordingly, menstruation.
There are some other possible causes of anovulation:
- poor nutrition;
- stress;
- excessive fatness or thinness;
- intense physical activity;
- environmental change.
These symptoms are often related. Anovulation, for example, can occur with regular and intense workouts that cause sudden weight loss. Of course, this does not mean the need to stop training, you need to adjust the exercises and slow down the pace. Physical activity must be adapted to capabilities that will develop over time and require additional effort.
Likewise, menstrual irregularities and anovulation are common in very thin women. The reason for this may be not only your physique, but also a stressful situation that forced you to lose weight. It is possible that we are talking about more severe forms of the disease, for example, anorexia or bulimia.
There can be many causes, so it is important to monitor symptoms.
How to correctly diagnose anovulation?
When a woman has not had her period for a long time or her cycle is very unstable and has large gaps, anovulation can be diagnosed very quickly. It is enough to undergo a short examination. The gynecologist will prescribe:
- progesterone test;
- gynecological ultrasound;
- folliculometry.
If the progesterone test does not give a clear result and the ultrasound does not show ovarian pathologies, additional studies are carried out:
- smear for a group of infections;
- comprehensive pelvic ultrasound;
- Ultrasound of the ovaries;
- Ultrasound of the corpus luteum;
- Ultrasound of the thyroid gland, since it also produces hormones that can affect reproductive function;
- a set of hormone tests: sex hormones, thyroid hormones and pituitary hormones are examined.
Diagnostics
In case of infertility, diagnosing anovulation is the first step to make the correct diagnosis and begin treatment.
Laboratory and instrumental research methods are used:
- blood chemistry;
- analysis of hormone concentrations;
- Ultrasound control and examination of the reproductive organs, thyroid and mammary glands.
If it is necessary to examine the hypothalamic-pituitary system, computed tomography or magnetic resonance imaging is prescribed.
Treatment of anovulation
Treatment consists of eliminating the reason why ovulation does not occur. It is important to understand that self-treatment of this pathology is completely excluded. The main advice: follow all the instructions of your doctor.
The gynecologist may prescribe:
- Hormonal drugs
. There are medications that stimulate the ovulatory process. They affect the maturation of follicles, increase estrogen levels and improve the possibility of an egg being released from the ovary. Such medications are prescribed only after all tests have been completed, since incorrect dosages lead to an even worse condition - ovarian hyperstimulation. It is also important to know that with hormonal stimulation of ovulation, several eggs can mature at once, which will lead to multiple pregnancies. - Antibiotics, antiviral drugs
. If infections are detected, they must be treated. Even with the restoration of the cycle and ovulation, the inflammatory process in the reproductive system will sooner or later lead to infertility. STIs are associated with problems such as obstruction of the tubes, hydrosalpinx - purulent inflammation of the ovary, in which it simply melts. - Surgery
. If anovulatory cycles are associated with organ pathologies, surgery is performed. - Lifestyle changes.
If cycle disturbances are due to external influences such as diet or lifestyle, treatment will include adjusting eating habits and mitigating exercise. You may also have to fight excess weight or, on the contrary, gain it.
Sometimes, the happiness of motherhood is very difficult for a woman, so try not to develop diseases and contact a gynecologist in a timely manner.
Polycystic ovary syndrome (anovulatory infertility type II)
If an ultrasound examination shows signs of polycystic ovary syndrome, they speak of anovulatory infertility type II. This form of pathology is often associated with hypoandrogenism and insulin resistance. Polycystic ovary syndrome is difficult to correct with medication. However, with properly selected hormonal therapy and duration of treatment, it is possible to expect a positive pregnancy outcome.
Treatment of this form of childlessness also involves a surgical operation - laparoscopic thermocautery. If the surgical intervention is successful, the likelihood of pregnancy increases significantly.
Length of the second phase of the cycle
The basal temperature chart is divided into the first and second phases. The division takes place where the ovulation line (vertical line) is marked. Accordingly, the first phase of the cycle is the segment of the graph before ovulation, and the second phase of the cycle is after ovulation.
The length of the second phase of the cycle is normally from 12 to 16 days, most often 14 days. In contrast, the length of the first phase can vary greatly and these variations are the individual norm. At the same time, in a healthy woman in different cycles there should be no significant differences in the length of the first phase and the second phase. The total length of the cycle normally changes only due to the length of the first phase.
One of the problems identified on the graphs and confirmed by subsequent hormonal studies is the failure of the second phase. If you measure your basal temperature over several cycles, following all the measurement rules, and your second phase is shorter than 10 days, this is a reason to consult a gynecologist . Also, if you regularly have sexual intercourse during ovulation, pregnancy does not occur and the length of the second phase is at the lower limit (10 or 11 days) , then this may indicate insufficiency of the second phase.
Middle line on the basal temperature chart
This line is drawn over 6 temperature values in the first phase of the cycle preceding ovulation.
This does not take into account the first 5 days of the cycle, as well as days on which the temperature could be affected by various negative factors (see rules for measuring temperature). This line does not allow any conclusions to be drawn from the graph and is for illustrative purposes only.
In what cases should you consult a gynecologist?
If you strictly follow the rules for measuring temperature and observe the problems described below on your chart in at least 2 cycles in a row, you can consult a doctor for additional examinations. of your gynecologist making diagnoses based solely on charts. What you need to pay attention to:
- anovulatory schedules
- regular cycle delays when pregnancy does not occur
- late ovulation and failure to become pregnant for several cycles
- controversial charts with unclear ovulation
- graphs with high temperature throughout the cycle
- graphs with low temperature throughout the cycle
- schedules with a short (less than 10 days) second phase
- graphs with a high temperature in the second phase of the cycle for more than 18 days, without the onset of menstruation and a negative pregnancy test
- unexplained bleeding or heavy discharge mid-cycle
- heavy menstruation lasting more than 5 days
- graphs with a temperature difference in the first and second phases of less than 0.4 degrees
- cycles shorter than 21 days or longer than 35 days
- charts with clearly defined ovulation, regular intercourse during ovulation and no pregnancy occurring for several cycles