Is it possible to teach our brain to artificially produce the “happiness hormone” - serotonin? This task is not easy, but feasible, because managing the delicate hormonal balance is within the power of any person. You just need to want it - and literally in 5 minutes you will be in a great mood and ready to “move mountains.” Hormones of pleasure and happiness will help you in this endeavor:
- serotonin - it is called the hormone of happiness, which gives a feeling of euphoria;
- dopamine – it is responsible for pleasure and reinforces positive experiences;
- Oxytocin - it gives rise to feelings such as love, tenderness and affection.
Most often, this “trinity” works in tandem, forming all our positive emotions. But now we will talk specifically about serotonin. Many people already know the name of this hormone of joy and happiness. But not everyone understands how it works. And even fewer people know how to regulate the level of this hormone in the body.
How to learn to manage serotonin?
This neurotransmitter, which regulates our mood, is not as simple as it might seem at first glance. Surely you think that serotonin is another hormone that is produced in the human brain. This is true, but only partly. Here are the hard facts: 95% of serotonin is produced in the intestines. And only 5% is in the brain.
So really, the path to our happiness lies through the stomach? And this is not entirely true. Serotonin is a complex chemical substance, a derivative of the amino acid tryptophan and, concurrently, the manager of our brain. This hormone is responsible for a wide range of processes both in the brain itself and throughout the human body:
- Serotonin in the brain helps nerve cells communicate better.
- The joy hormone affects sleep and appetite, muscle condition and body function, character and mood.
- The most important neurotransmitter affects cognitive functions (attention, memory, communication, etc.).
- Serotonin is responsible for normal blood clotting and stable functioning of the circulatory system.
- The joy hormone helps us experience interest and attraction to the opposite sex.
Catecholamines
In the middle of the twentieth century, J. Shildkraut (1961) formulated the so-called catecholamine theory of the origin of depression. This theory suggested dysregulation of the brain system sensitive to norepinephrine as one of the important links in the pathogenesis of depression during depression (Praag H., 1994; Ashmarin I.P., et al., 1999).
It is now known that the content of norepinephrine in brain cells is controlled by special endings of the nerve cell - presynaptic adrenergic receptors. Stimulation of these receptors inhibits the release of norepinephrine, which in turn leads to its deficiency in the synapse and a decrease in neurotransmission. Blockade of these receptors by antidepressants, on the contrary, leads to an increase in the release of norepinephrine.
Fundamental studies of the reticular formation of the brain have shown that antidepressants, the action of which is aimed at changing the content of norepinephrine, have a general activating or psychostimulating effect. These drugs maintain the level of wakefulness, help improve the processes of perception, thinking, memory and increase concentration. However, despite the fact that taking antidepressants increases norepinephrine levels almost immediately, the clinical effect of the drug appears much later.
According to the catecholamine hypothesis of J. Schildkraut. (1978) the development of depression, especially endogenous, is caused by a decrease in the content of catecholamines, mainly norepinephrine, in certain brain structures. It was also assumed that the functional activity of noradrenergic systems during depression can be indirectly assessed by the content in the urine of such a metabolite of norepinephrine as MOPEG (3-methoxy-4-hydroxyphenylethylene glycol).
Based on a number of observations (Schildkraut J., 1978, Beckmann H., Goodwin F., 1980), it was suggested that the level of MOEFG can serve as a predictor of the effectiveness of therapy for various antidepressants. Depressed patients with lower MOPEG levels may benefit from imipramine and desipramine, but are resistant to amitriptyline.
It has been suggested that in this group of patients the primary predominant disorder is norepinephrine metabolism. On the contrary, depressed patients with high daily levels of MOFDH excretion respond better to amitriptyline therapy. At the same time, it was shown that in mentally healthy people the daily excretion of MOPEG fluctuates four times, and these fluctuations overlap the parameters of the shifts noted in patients suffering from depression (Hollister L. et al., 1978). In addition, it was found that during the period of clinical remission, the shifts in catecholamines found in patients with depression do not normalize, and even during severe depression, the content of catecholamines may be within normal limits. (Cazzulo S., Sacchetti E. et al., 1982).
Based on data obtained by J. Costa, E. Silva (1980), a theory arose suggesting the presence of two variants of the occurrence of depression, one associated with the depletion of norepinephrine and more sensitive to treatment with some antidepressants (desimipramine or imipramine) and the other associated with a deficiency of serotonin and responding to therapy with other drugs (amitriptyline). It was assumed that antidepressants exert their therapeutic effect by facilitating the transmission of both norepinephrine and serotonin (Haefely W., 1985).
Recent research in the field of brain physiology has shown that the norepinephrine-sensitive system in the brain has a pronounced influence on the serotonin-sensitive system. It turned out that nerve cells sensitive to norepinephrine control the rate of serotonin release by influencing the endings of neurons located on the bodies of serotonergic neurons. An increase in the excitability of serotonergic neurons, in turn, increases the release of serotonin in nerve endings (De Boer T., et al., 1994).
What happens if serotonin levels are disrupted?
Very often we hear about how low levels of the happiness hormone are bad. But the human body is harmed not only by a deficiency, but also by an excess of serotonin. In other words, any disruption in the production of this neurotransmitter provokes a whole list of negative consequences and conditions:
- bad mood and chronic depression;
- a sharp decrease in sexual desire;
- problems with digestion and sleep;
- cravings for sweets and weight gain;
- decreased activity.
The problems listed above are just the tip of the iceberg. The work of serotonin, based on two substances - the amino acid tryptophan and its derivative 5-hydroxytryptophan, is very complex. Sometimes the hormonal imbalance is so pronounced that specialists have no choice but to prescribe antidepressants - serotonin reuptake inhibitors.
Dopamine
The pathogenesis of depression may also be based on a lack of another biological substance, which to some extent is a precursor of norepinephrine - dopamine. It is assumed that it takes part in the regulation of the function of the motor sphere, has a psychostimulating effect and is responsible for the formation of certain behavior (Mosolov S.N., 2002). Proof of this hypothesis is the ability of the drug L-DOPA, which is a precursor of dopamine and norepinephrine, to promote the transition of depression to a state of increased activity (Bunney W. et al., 1970; Van Praag H., 1977). When using L-DOPA in patients suffering from depression, a positive effect in the form of changes in activity was often noted. So, in particular, R.Ya. Bovin, I.O. Aksenova (1982), when using L-DOPA in treatment-resistant depressive patients, noted a positive effect in the form of increased psychomotor activity in 25% of cases.
In addition, taking medications that lower dopamine levels, such as rauwolfia, can lead to depression. A decrease in dopamine levels is observed in a number of neurological and somatic diseases, also accompanied by depression, such as Parkinson's disease.
But is it worth using “heavy artillery” right away?
We know that antidepressants work quickly. But we also know that they have a lot of disadvantages, the most important of which are addiction and various side effects that can seriously undermine already weakened health. The image below shows how hard antidepressants work. It’s worth thinking about whether such radical measures are necessary???
Is there a safe alternative to pills? Eat. This is the right way of life and the ability to look at the world positively. The “recipe” for your happiness will not consist of a set of drugs, but of completely different components:
- Nutrition. Healthy foods contain everything you need to produce the hormone serotonin in sufficient quantities. Give up fast carbohydrates and junk food in favor of healthy carbohydrates and foods containing tryptophan. Serotonin “grows” on variety: grains and cereals, vegetables and fruits, eggs and dairy products, lean meat and fatty fish, nuts, seeds and dried fruits.
The image shows foods rich in tryptophan.
- Movement. Scientists have repeatedly proven that any physical activity quickly (literally in 30-40 minutes) increases the level of serotonin in the blood. Dancing, walking, swimming, cycling, morning jogging - there are many activities, you don’t have to go to the gym. But there is one “but”: you need to be active constantly, and not from time to time.
- Sleep and meditation. A happy person knows how to “switch off” in time and let go of negativity from his life. Sometimes this is difficult to do. And then meditation and healthy sleep come to the rescue, during which the level of the happiness hormone also increases. Train yourself to go to bed and get up at the same time - and you will not have problems with serotonin.
- More sun. In our climate, sunny days are a rare occurrence. But even on a cloudy day, our skin still receives its portion of ultraviolet radiation. This means that the answer to the question “how to increase serotonin” is simple: walk more often. Walking will give the body maximum happiness hormone and beneficial vitamin D.
- Omega-3. It is difficult for us to maintain an optimal level of essential polyunsaturated fatty acids, which are found in excess only in the meat of fatty fish from the northern seas. But now there is already a worthy alternative - dietary supplement complexes with Omega-3. Such supplements are a real “delicacy” for the brain and prevent a number of diseases.
- Pleasure. Serotonin is responsible for pleasure and enjoyment. How can you get more of these emotions? There are a lot of ideas: for example, falling in love, finding a new exciting hobby, meeting friends more often, spending more time with loved ones. The more bright emotions and positivity there are in your life, the higher the level of the happiness hormone will be.
- "Helpers." Unfortunately, even with proper nutrition, it is not possible to obtain from food 100% of the substances necessary to nourish the brain and body. And then “serotonin in tablets” comes to the rescue. These are special plant vitamin complexes that help the happiness hormone be produced correctly and in the required volume.
The role of serotonin receptors in the motor-evacuation function of the gastrointestinal tract
The main classes of receptors involved in the regulation of motor-evacuation function of the gastrointestinal tract (GIT) are cholinergic, adrenergic, dopaminergic, serotonin, motilin and cholecystokinin. Drugs used for depressive and anxiety disorders, panic attacks and other autonomic dysfunctions act on the same receptors that are responsible for the motor-evacuation function of the gastrointestinal tube. Regulation of smooth muscle activity and intestinal motility occurs at several levels. Hormones and neurotransmitters are the dominant components that directly or indirectly affect smooth muscle cells. The postprandial endocrine response includes the production of insulin, neurotensin, cholecystokinin (CCK), gastrin, glucagon-like peptides (GLP-1 and GLP-2), glucose-dependent insulinotropic polypeptide (GIP, formerly known as gastric inhibitory peptide) [1], effect data neurotransmitters and hormones are presented in table. 1. For example, CCA is secreted in the proximal small intestine and directly influences the contraction of gallbladder muscle cells and the neuromediated relaxation of sphincter of Oddi cells, which is mediated through the GIP neuromuscular junction.
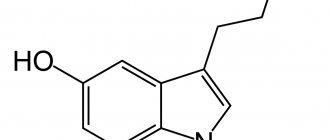
In this article, special attention is paid to serotonergic receptors, which are one of the important regulators of intestinal motility. Serotonin, or 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter that is the main mediator in the physiology of a person’s psychological state and mood, as well as one of the regulators of vascular function and gastrointestinal motility. 5-HT is known to be present in platelets, the gastrointestinal tract, and the central nervous system of humans and animals [2–5]. Serotonin is produced in the human body from the amino acid tryptophan supplied with food - since it is precisely this that is needed for the direct synthesis of serotonin in synapses; the second pathway for the production of serotonin is associated with the supply of glucose with carbohydrate foods, which stimulates the release of insulin into the blood, followed by protein catabolism in tissues, which also leads to an increase in the level of tryptophan in the blood.
Based on biochemical and pharmacological criteria, 5-HT receptors are divided into seven main subtypes, five of which are found in enterochromaffin neurons, enterochromaffin (EC) cells and gastrointestinal smooth muscle: 5-HT1, 5-HT2, 5-HT3, 5 -HT4 and 5-HT7 [6, 7]. About 80% of the total number of 5-HT receptors are located in intestinal Echo cells, where they participate in intestinal motility through several subtypes of 5-HT receptors [8, 9]. With the exception of 5-HT3 receptors, a gated ion channel ligand, all 5-HT receptors bind to G protein receptors, which activate second-cascade intracellular reactions, stimulating excitatory or inhibitory responses in the gastrointestinal tract [10]. Serotonin has well-studied effects on intestinal motility, secretion and sensitivity through central and peripheral neurotransmitter pathways, making it a key pharmacological agent used in the treatment of gastrointestinal motility disorders [11]. Serotonin is released from EC cells in response to chemical or mechanical stimulation of the mucosa [12] or in response to experimental models of stress [13]. Serotonin is synthesized and stored not only in EC cells (90%), but also in intestinal neurons (10%). As mentioned above, 5-HT is released into the blood after eating and in response to changes in pressure in the intestinal wall, as well as when exposed to harmful stimuli [13], and then enters the intestinal lumen and further into its walls from the basolateral depot of ECH cells [14]. 5-HT stimulates the circular and longitudinal muscles of the stomach, duodenum and jejunum [15]. The strategic location of EC cells in close proximity to sensory nerve endings of the intestinal mucosa, interganglionic neurons, and synapses of motor excitatory and inhibitory neurons is important. Serotonin increases the contraction amplitude of the muscles of the stomach, duodenum, jejunum and ileum [16]. In the small intestine, 5-HT stimulates circular muscle contractions during the first manometric phase, the resulting contractions spread, become more frequent and activate fast motor complexes [17]. In the colon, serotonin stimulates motility along the entire length, causing phasic contractions, but not giant motor complexes [18]. Intestinal smooth muscle rhythmic oscillations are determined by the spontaneous activity of interstitial cells of Cajal, which act as a pacemaker for cells in the gastrointestinal tract [19–22]. The enteric nervous system (ENS) consists of semi-autonomous effector systems that are connected to the central autonomic system. When serotonin is released from enterochromaffin cells, vagal reflexes are initiated - peristaltic, excretory, vasodilatory, nociceptive. The parasympathetic and sympathetic divisions of the autonomic nervous system form the CNS through afferent and efferent connections. The ongoing bidirectional relationship of the brain-gut reflex arc involving 5-HT has a significant impact on effector systems. Impaired 5-HT transmission can lead to both intestinal and extraintestinal manifestations of irritable bowel syndrome (IBS) [23].
The degree of participation of various 5-HT in the functional peristaltic activity of the gastrointestinal tract is divided as follows - 5-HT3 - 65%, 5-HT4 - 85% and 5-HT7 - 40%. When combined, antagonists of these receptors, given in pairs, are able to reduce intestinal peristaltic activity by approximately 16% (5-HT3 + 5-HT4), 70% (5-HT3 + 5-HT7) and 87% (5-HT4 + 5-HT7), and the simultaneous administration of all three antagonists inevitably blocks all peristaltic activity. Thus, 5-HT receptors play a key role in modulating intestinal motility by simultaneously blocking three receptors and inhibiting peristaltic activity. Among the 5-HT receptors of the gastrointestinal tract, the 5-HT4 subtype is the most functionally important for peristalsis, and the 5-HT3 and 5-HT7 receptors play a slightly less active role in this process, as reflected in Table. 2 and in Fig. 1 [24].
5-HT4 agonists became available with the introduction of metoclopramide in 1964. This drug is a dopamine D2 and 5-HT3 receptor antagonist and a 5-HT4 receptor agonist and is still widely used throughout the world. Its success has led to the development of alternative molecules that do not affect D2 receptors, thereby eliminating adverse events such as akathisia and extrapyramidal movement disorders.
Serotonin receptors, in particular 5-HT3 and 5-HT4, are involved in sensory and reflex responses to stimuli in gastrointestinal disorders, causing manifestations such as vomiting, constipation or diarrhea, eating disorders, abdominal pain, altered sensorimotor reflexes [25 ]. It has been suggested that selective serotonin reuptake inhibitors (SSRIs) may affect 5-HT3 receptor function and may also improve symptoms of IBS and comorbid depression in patients. According to a number of studies and reviews [26–29], tricyclic antidepressants (amitriptyline, Melipramine), antidepressants of a number of SSRIs, such as fluoxetine, paroxetine, citalopram, clomipramine, litoxetine, trazodone, and a number of selective serotonin and norepinephrine reuptake inhibitors (SSRIs) ( duloxetine) improves IBS symptoms. Long-term side effects of this therapy are common to antidepressant treatment and include anticholinergic, serotonergic, sedative, antihistamine, and alpha-adrenergic effects. These effects must be taken into account when choosing a treatment approach, since the drugs described above affect intestinal motility, the patient's bowel function should also be taken into account when choosing serotonergic drugs (Fig. 2) [30].
As stated previously, the 5-HT1, 5-HT3, and 5-HT4 receptor subtypes play important roles in the motor, sensory, and secretory functions of the gastrointestinal tract. Drugs that directly affect 5-HT receptors, unlike tricyclic antidepressants and SSRIs, modulate 5-hydroxytryptamine (5-HT) by binding to 5-HT receptors; their characteristics are shown in Table. 3. Intestinal functions of 5-HT receptors are associated with smooth muscle, increased bowel movements, and decreased intestinal transit time [31, 32]. Blockade of 5-HT3 receptors, in particular by antiemetics such as ondansetron, leads to constipation [33]. Over the past decade, the 5-HT3 receptor blockers alosetron and silansetron have been developed and tested for IBS-D (IBS with diarrhea). A recent systematic review and meta-analysis of 11 randomized controlled trials (RCTs) comparing these two 5-HT3 antagonists with placebo found a beneficial effect [34]. However, a number of rare side effects, including ischemic colitis and severe constipation, led to the suspension of alosetron production and research on silansetron [35]. Alosetron is currently available only under strict indications (in the US) for patients with severe refractory IBS with diarrhea who have not responded to first or second line therapy.
5-HT4 agonists have proven their therapeutic potential for the treatment of patients with gastrointestinal motility disorders. Drugs that lack selectivity for 5-HT4 receptors have limited clinical success in gastroenterological practice. For example, in addition to their affinity for 5-HT4 receptors, drugs such as cisapride and tegaserod also have marked affinity for other receptors, channels, or transmitter proteins. The adverse cardiovascular events observed with these agents are due to their nonselectivity and crossover effects. A systematic review and meta-analysis found that tegaserod is superior to placebo in the treatment of constipation, including IBS. Most of the studies related to tegaserod were conducted in women, and as a result, the drug was initially approved for the treatment of IBS-C (IBS with constipation) only in women. However, marketing of tegaserod was also suspended when data on a possible increase in cardiovascular and cerebrovascular events with the drug were reported [6].
An important event in clinical pharmacology was the discovery of a selective ligand (ligand, from Latin ligare - to bind, an atom, ion or molecule associated with a certain center (acceptor), the term is used in biochemistry to designate agents that combine with biological acceptors - receptors, immunoglobulins and etc.) to the 5-HT4 receptor - prucalopride. The selectivity of this new drug significantly distinguishes it from older generations of alternative drugs by minimizing the potential for side effects. In addition, the concept of searching for similar ligands opens up broad opportunities for further drug development and the creation of agonist-specific effects in various types of cells, tissues or organs. The selective 5-HT4 receptor agonist prucalopride is an innovative drug with an attractive safety profile for the treatment of patients suffering from hypomotility gastrointestinal disorders [36]. Prucalopride has high affinity and selectivity for 5-HT4 receptors in the gastrointestinal tract. During the existence of the drug prucalopride, several large and long-term studies were conducted, which made it possible to fully evaluate the risks and benefits of using prucalopride for chronic constipation [36–38]. Overall, prucalopride was associated with consistent and significant improvements in patient satisfaction with their treatment, as assessed by the Patient Assessment of Constipation Quality of Life questionnaire (PAC-QOL). The proportion of participants receiving prucalopride 2 mg daily who reported an improvement of ≥ 1 point on the PAC-QOL 5-point subscale was 45.3%, compared with 21.3% of those receiving placebo (p ≤ 0.001), but the response rate in almost all studies was less than 50%. The other trials, PRU-USA-11 and PRU-USA-13, found no significant difference between prucalopride and placebo at all surrogate points. The overall incidence of adverse events was statistically significantly higher in patients receiving prucalopride (72%) compared with patients receiving placebo (59%) (hazard ratio (RR) 1.21, 95% confidence interval (CI): 1.06 , 1.38). The adverse events most commonly reported by patients receiving prucalopride were headache (up to 30%), nausea (up to 24%), diarrhea (up to 5%), abdominal pain and flatulence (up to 23%), dizziness (up to 5 %) and upper respiratory tract infections [39]. R. Cinca et al. compared the effectiveness, safety and impact on quality of life of macrogol and prucalopride in 240 women with chronic constipation for whom other laxatives did not provide adequate relief. In this study, macrogol was more effective than prucalopride for the treatment of chronic constipation and was better tolerated [40]. As a result, we can conclude that prucalopride can be prescribed by a doctor experienced in the treatment of chronic constipation to women from 18 to 75 years of age if other laxatives have not been effective in their treatment.
It is important to know that patients do not always have a deficiency of serotonin; in some cases, the doctor may encounter an excess of it. In restless gastroenterological patients who have elevated serotonin levels, aerophagia develops, which causes an increase in the air bubble in the stomach and leads to irritation of the receptor apparatus [41]. Elevated levels of serotonin cause frequent nausea and vomiting due to activation of the vagus nerve, diarrhea or spastic constipation, gastrointestinal panic attacks, headache, tremor, hyperhidrosis, agitation and anxiety, palpitations, unstable blood pressure, insomnia.
Serotonin plays an important role not only in the regulation of motility and secretion in the gastrointestinal tract, enhancing its peristalsis and secretory activity, but is also a growth factor for some types of symbiotic microorganisms and enhances bacterial metabolism in the colon. The colon bacteria themselves also make some contribution to the intestinal secretion of serotonin, since many species of commensal bacteria have the ability to decarboxylate tryptophan. With dysbiosis and a number of other diseases of the colon, the production of serotonin by the intestines is significantly reduced. The massive release of serotonin from dying cells of the gastric and intestinal mucosa under the influence of cytotoxic chemotherapy is one of the causes of nausea and vomiting, as well as diarrhea during chemotherapy for malignant tumors [42, 43].
It is difficult to overestimate the role of serotonin in the human body. In the front part of the brain, under the influence of serotonin, areas responsible for the process of cognitive activity are stimulated, and an increase in serotonergic activity creates a feeling of uplift in the cerebral cortex. Serotonin entering the spinal cord has a positive effect on motor activity and muscle tone; this state can be characterized by the phrase “I can move mountains.” In addition to mood, serotonin is “responsible” for self-control or emotional stability. Serotonin controls the sensitivity of brain receptors to stress hormones adrenaline and norepinephrine. In people with low serotonin levels, the slightest trigger triggers a massive stress response. Some researchers believe that the dominance of an individual in the social hierarchy is due precisely to the high level of serotonin [42, 43].
Conclusion
When food enters the body, including food containing tryptophan, the production of serotonin increases, which improves mood. The brain quickly grasps the connection between these phenomena and, in the case of depression (serotonin starvation), immediately “demands” additional food intake with tryptophan or glucose. The richest foods in tryptophan are those that consist almost entirely of carbohydrates, for example, bread, bananas, chocolate, figs, dried apricots, dates, raisins, watermelons, etc. The listed products have long been known as regulators of intestinal motility. Their nutritional deficiency leads to depression and gastrointestinal problems, which can often be observed in people following a strict low-calorie diet. For this reason, before prescribing medications to a patient that increase serotonin levels, it is necessary to clarify the cause of its deficiency. Knowledge of the details of the structure of serotonin receptors will undoubtedly find application in treating patients with non-cardiotoxic serotonin analogues or drugs that increase serotonin levels, which will perform their healing function and will be pleasant in all respects, for example, such as chocolate [44]. Drugs that increase the level of serotonin in the synaptic cleft and enhance its effects belong to the group of antidepressants. Today they are among the most prescribed medications by general medical practitioners in many countries around the world, including Europe and North America. Timely prescription of antidepressants, both in monotherapy and in treatment regimens for various diseases, can increase the effectiveness of treatment of the underlying disease and improve the quality of life of patients, especially in gastroenterological patients.
Literature
- Medhus A.W., Sandstad O., Naslund E. at al. The influence of the migrating motor complex on the postprandial endocrine response // Scand J Gastroenterol. 1999. 34. R. 1012–1018.
- Buchheit KH, Engel G., Mutschler E., Richardson B. Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea pig ileum. Evidence for two distinct release mechanisms // Naunyn Schmiedebergs Arch Pharmacol. 1985. 329. R. 36–41.
- Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection // Am J Gastroenterol. 2000. 95. R. 2698–2709.
- Woollard DJ, Bornstein JC, Furness JB Characterization of 5-HT receptors mediating contraction and relaxation of the longitudinal muscle of the guinea pig distal colon in vitro // Naunyn Schmiedebergs Arch Pharmacol. 1994. 349. R. 455–462.
- Yamano M., Ito H., Miyata K. Species differences in the 5-hydroxytryptamine-induced contraction in the isolated distal ileum // Jpn J Pharmacol. 1997. 74. R. 267–274.
- De Maeyer JH, Lefebvre RA, Schuurkes JA 5-HT4 receptor agonists: similar but not the same // Neurogastroenterol Motil. 2008. 20. R. 99–112.
- Hannon J., Hoyer D. Molecular biology of 5-HT receptors // Behav Brain Res. 2008. 195. R. 198–213.
- Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection // Am J Gastroenterol. 2000. 95. R. 2698–2709.
- Berger M., Gray JA, Roth BL The expanded biology of serotonin // Annu Rev Med. 2009. 60. R. 355–366.
- Hannon J., Hoyer D. Molecular biology of 5-HT receptors // Behav Brain Res. 2008. 195. R. 198–213.
- Crowell MD Role of serotonin in the pathophysiology of the irritable bowel syndrome // Br J Pharmacol. 2004. 141. R. 1285–1293.
- Gershon MD Plasticity in serotonin control mechanisms in the gut // Curr Opin Pharmacol. 2003. 3. R. 600–607.
- Bearcroft CP, Perrett D., Farthing MJ Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study // Gut. 1998. 42. R. 42–46.
- Hansen MB Small intestinal manometry // Physiol Res. 2002. 51. R. 541–556.
- Fishlock DJ, Parks AG, Dewell JV Action of 5-hydroxytryptamine on the human stomach, duodenum, and jejunum in vitro // Gut. 1965. 6. R. 338–342.
- Hopkinson GB, Hinsdale J., Jaffe BM Contraction of canine stomach and small bowel by intravenous administration of serotonin. A physiological response? // Scand J Gastroenterol. 1989. 24. R. 923–932.
- Hansen MB, Gregersen H., Husebye E., Wallin L. Effect of serotonin and ondansetron on upper GI manometry in healthy volunteers // Neurogastroenterol Motil. 2000. 12. R. 281.
- Boerckxstaens GE, Pelckmans PA, Rampart M. at al. Pharmacological characterization of 5-hydroxytryptamine receptors in the canine terminal ileum and ileocolonic junction // J Pharmacol ExpTher. 1990. 254. R. 652–658.
- Alberti E., Mikkelsen HB, Larsen JO, Jimenez M. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid- and distal-colon of the rat // Neurogastroenterol Motil. 2005. 17. R. 133–147.
- Sanders KM A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract // Gastroenterology. 1996. 111. R. 492–515.
- Thomsen L., Robinson T.L., Lee J.C. at al. Interstitial cells of Cajal generate a rhythmic pacemaker current // Nat Med. 1998. 4. R. 848–851.
- Park SY, Je HD, Shim JH, Sohn UD Characteristics of spontaneous contraction in the circular smooth muscles of cat ileum // Arch Pharm Res. 2010. 33. R. 159–165.
- Crowell MD Role of serotonin in the pathophysiology of the irritable bowel syndrome // Br J Pharmacol. 2004. 141 (8). R. 1285–1293.
- Balestra B., Vicini R., Pastoris O. at al. 5-HT receptors and control of intestinal motility: expression and hierarchical role // Poster Session, Bologna. 2011.
- Read NW, Gwee KA The importance of 5-hydroxytryptamine receptors in the gut // Pharmacol Ther. 1994. Apr-May; 62 (1–2). R. 159–173.
- Lucchelli A., Santagostino-Barbone MG, Barbieri A. at al. The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors // Br J Pharmacol. 1995. Mar; 114(5). R. 1017–1025.
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis // Gut. 2009. Mar; 58(3). R. 367–378.
- Friedrich M., Grady SE, Wall GC Effects of antidepressants in patients with irritable bowel syndrome and comorbid depression // Clin Ther. 2010. July; 32(7). R. 1221–1233.
- Chial HJ, Camilleri M, Burton D at al. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health // Am J Physiol Gastrointest Liver Physiol. 2003. 284. G130-G137.
- Turvill JL, Connor P., Farthing MJ The inhibition of cholera toxin-induced 5-HT release by the 5-HT (3) receptor antagonist, granisetron, in the rat // Br J Pharmacol. 2000. 130. R. 1031–1036.
- Ruckebusch Y., Bardon T. Involvement of serotonergic mechanisms in initiation of small intestine cyclic motor events // Dig Dis Sci. 1984. 29. R. 520–527.
- Haus U., Spath M., Farber L. Spectrum of use and tolerability of 5-HT3 receptor antagonists // Scand J Rheumatol Suppl. 2004. 119. R. 12–18.
- Ford AC, Brandt. LJ, Young C. at al. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: Systematic review and metaanalysis // Am J Gastroenterol. 2009. 104. R. 1831–1843.
- US Food and Drug Administration. Glaxo Wellcome withdraws irritable bowel syndrome medication // FDA Consum. 2001. 35. R. 3.
- Johanson JF, Drossman DA, Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation // Aliment. Pharmacol. 2008. 27. R. 685–696.
- Camilleri M., Kerstens R., Rykx A., Vandeplassche L. A Placebo-Controlled Trial of Prucalopride for Severe Chronic Constipation // N Engl J Med. 2008. 358. R. 2344–2354.
- Tack J., van Outryve M., Beyens G., Kerstens R., Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives // Gut. 2009; 58: 357–565.
- Quigley E.M., Vandeplassche L., Kerstens R., Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation a 12-week, randomized, double-blind, placebo-controlled study // Aliment Pharmacol Ther. 2009; 29: 315–328.
- https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2012–7/prucalopride.pdf.
- Cinca R., Chera D., Gruss HJ, Halphen M. Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation - a comparison in a controlled environment // Aliment Pharmacol Ther. May 2013; 37(9). R. 876–886.
- Burov N. E. Nausea and vomiting in clinical practice (etiology, pathogenesis, prevention and treatment) // Russian Medical Journal. 2002. No. 16. pp. 390–395.
- Barinov E.F., Sulaeva O.N. The role of serotonin in the physiology and pathology of the gastrointestinal tract // RZHGGK. 2012. T. 21. No. 2. P. 4–13.
- Ashmarin I. P., Eshchenko N. D., Karazeeva E. P. Neurochemistry in tables and diagrams. M.: “Exam”, 2007. 143 p.
- Palczewski K., Kiser PD As good as chocolate // Science. 2013. 340. R. 562–563.
E. Yu. Plotnikova1, Doctor of Medical Sciences, Professor O. A. Krasnov, Doctor of Medical Sciences, Professor
State Budgetary Educational Institution of Higher Professional Education Kemerovo State Medical Academy of the Ministry of Health of the Russian Federation, Kemerovo
1 Contact information
How do vitamins for a good mood work?
Unlike antidepressants, which inhibit serotonin reuptake, plant-based vitamins act differently—more gently. They bring all neurotransmitters into balance and help the happiness hormone be produced in the right quantity without causing serious harm to the human body:
- fight fatigue and irritability;
- increase memory, attention and concentration;
- eliminate insomnia and other sleep disorders;
- protect against depression and nervous disorders;
- restore interest in life and normalize appetite.
Constant stress at work and at home does not allow you to always be at the peak of activity. But now this problem can be easily solved: just one capsule of herbal vitamins a day - and it will be much easier for you to cope with the challenges that this world has prepared for you. Forgetfulness, absent-mindedness and apathy will be replaced by cheerfulness and a positive mood.
Endorphins and other neurotransmitters
In addition to mediators, depression may cause changes in endorphins - neuropeptides, biologically active substances that have the properties of a hormone and a mediator at the same time. Endorphins are responsible for a person's sensitivity to pain. Low levels of endorphins in dysthymia explain poor pain tolerance in people suffering from depression.
In depressive spectrum disorders, disorders of synaptic transmission are detected, mainly related to the inhibitory GABAergic systems of the brain (a decrease in gamma-aminobutyric acid is noted during depression). Gamma-aminobutyric acid released into the blood reduces anxiety levels. It also takes part in regulating the flow of nerve impulses by blocking the release of other neurotransmitters, such as dopamine and norepinephrine. As a result, there is a disorganization of the interaction of nerve cells that process sensory (extra- and interoceptive) information and integrate motor and regulatory activity. In this case, various mental disorders arise with corresponding neurological and autonomic manifestations (Eccles J., 1971; Guselnikov V.I., Iznak A.F., 1983; Glezer V.D., 1985).
With depression, a change in the concentration of biologically active substances occurs not only between nerve cells, but also inside neurons. These substances in the wall of the nerve cell are broken down into smaller components, which increase the activity of neurons by changing the direction of movement of transmitters to the center of the neuron, to its nucleus.
Return to Contents
Managing happiness hormones is easy!
The secret to the effectiveness of herbal vitamins for mood is in the ideal composition. There is nothing superfluous in them besides the active components - extracts of medicinal herbs. For example, these could be:
- St. John's wort and clover grass;
- Rhodiola rosea and valerian;
- volodushka and muira puama bark.
Such vitamins, even with long-term use, often turn out to be more effective and safe in comparison with synthetic drugs and antidepressants, which cause addiction, withdrawal effects, drowsiness and other side effects.
Now you know how to properly increase the level of serotonin in the body so that a positive result is not long in coming. Allow the happiness hormone to be produced in full - and your life will become bright, and every day will bring maximum benefit and pleasure.