Release form
Powder for the preparation of a solution for oral administration (for children) is heterogeneous, from white to pale yellow, with the smell of lemon; dissolves in 125 ml of hot water to form a cloudy solution from yellow to yellow-green in color, without foam on the surface, with the smell of lemon; There may be some slight sediment. 1 pack paracetamol 300 mg phenylephrine hydrochloride 5 mg ascorbic acid 20 mg Excipients: sodium saccharinate - 21.5 mg, sodium cyclamate - 31.5 mg, citric acid - 340 mg, sodium citrate - 215 mg, corn starch - 100 mg, sucrose - 1862.5 mg, flavoring lemon 610399E - 100 mg, curcumin dye (E100) - 3.5 mg, colloidal silicon dioxide - 1 mg. 3 g - laminate bags (10) - cardboard packs.
special instructions
The drug should not be taken simultaneously with other drugs containing paracetamol, as well as other non-narcotic analgesics (metamizole sodium), NSAIDs (acetylsalicylic acid, ibuprofen), with other drugs to relieve symptoms of colds and flu, sympathomimetics (such as decongestants, appetite suppressants , amphetamine-like psychostimulants), barbiturates, anticonvulsants, rifampicin and chloramphenicol. When conducting tests to determine uric acid and blood glucose levels, you should inform your doctor about the use of Coldrex® Junior, because the drug may distort the results of laboratory tests assessing the concentration of glucose and uric acid. Before taking the drug, you should consult a doctor if: - taking metoclopramide, domperidone (used to eliminate nausea and vomiting) or cholestyramine, used to reduce the concentration of cholesterol in the blood; - taking medications to reduce blood clotting (for example, warfarin); - following a low sodium diet - each sachet contains 0.12 g of sodium. To avoid toxic liver damage, paracetamol should not be combined with drugs containing ethanol. Impact on the ability to drive vehicles and machinery If dizziness occurs, the patient should refrain from driving or other potentially hazardous activities that require concentration and speed of psychomotor reactions.
Coldrex Junior lemon pore N10 (Nizhpharm)
Paracetamol, when taken for a long time, enhances the effect of indirect anticoagulants (warfarin and other coumarins), which increases the risk of bleeding. Occasional administration of a single dose of the drug does not have a significant effect on the effect of indirect anticoagulants. Inducers of microsomal oxidation enzymes in the liver (barbiturates, diphenin, carbamazepine, rifampicin, zidovudine, phenytoin, ethanol, flumecinol, phenylbutazone and tricyclic antidepressants) increase the risk of hepatotoxicity in overdoses and concomitant use with paracetamol. Microsomal oxidation inhibitors (cimetidine) reduce the risk of hepatotoxicity. Paracetamol reduces the effectiveness of diuretics. Metoclopramide and domperidone increase, and cholestyramine reduces the rate of absorption of paracetamol. Paracetamol enhances the effects of MAO inhibitors, sedatives, ethanol. The simultaneous use of paracetamol and alcoholic beverages increases the risk of developing liver damage and acute pancreatitis. Phenylephrine when taken with MAO inhibitors can lead to increased blood pressure. Phenylephrine reduces the effectiveness of beta-blockers and other antihypertensive drugs (including debrisoquine, guanethidine, reserpine, methyldopa), increases the risk of developing arterial hypertension and disorders of the cardiovascular system. Tricyclic antidepressants (eg, amitriptyline) enhance the sympathomimetic effects of phenylephrine and may increase the risk of cardiovascular side effects. The simultaneous administration of halothane with phenylephrine increases the risk of developing ventricular arrhythmia. Concomitant use of the drug and ergot alkaloids (ergotamine and methylsergide) increases the risk of ergotism. Antidepressants, antiparkinsonian drugs, antipsychotic drugs, phenothiazine derivatives increase the risk of developing urinary retention, dry mouth, and constipation. Concomitant use of glucocorticosteroids with phenylephrine increases the risk of developing glaucoma. Concomitant use of digoxin and other cardiac glycosides increases the risk of developing cardiac arrhythmias and heart attack. Concomitant use of phenylephrine with sympathomimetic amines may increase the risk of adverse cardiovascular effects. Ascorbic acid, when used simultaneously with iron preparations, due to its restorative properties, converts ferric iron into divalent iron, which helps improve its absorption. When used concomitantly, ascorbic acid increases iron excretion in patients receiving deferoxamine. When used simultaneously with barbiturates and primidone, the excretion of ascorbic acid in the urine increases. Ascorbic acid in high doses can reduce urine pH, which, when used simultaneously, reduces the tubular reabsorption of amphetamine and tricyclic antidepressants. When used simultaneously, acetylsalicylic acid reduces the absorption of ascorbic acid by about a third. When used simultaneously with warfarin, the effects of warfarin may be reduced. When used simultaneously with tetracycline, the excretion of ascorbic acid increases.
Contraindications
- hypersensitivity to the components of the drug; - severe liver diseases; - severe kidney disease; — diseases of the hematopoietic system; - thyrotoxicosis; - arterial hypertension; - heart disease (severe stenosis of the aortic mouth, acute myocardial infarction, tachyarrhythmias); - prostatic hyperplasia; - angle-closure glaucoma; - diabetes; - genetic absence of glucose-6-phosphate dehydrogenase; - sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption, because the drug contains sucrose; - simultaneous use of tricyclic antidepressants, beta-blockers, MAO inhibitors and a period of up to 14 days after their discontinuation; - children up to 6 years of age. The drug should be used with caution in cases of benign hyperbilirubinemia, pheochromocytoma, and peripheral vascular diseases (Raynaud's syndrome).
Coldrex Junior powder for intravenous doses for children pack 3g No. 10
Compound
Paracetamol 300 mg, phenylephrine hydrochloride 5 mg, ascorbic acid 20 mg. Excipients: sodium saccharinate, sodium cyclamate, citric acid, sodium citrate, corn starch, sucrose, lemon flavor (610399E), curcumin dye (E100), colloidal silicon dioxide.
Pharmacokinetics
Coldrex Junior Hot Drink is a combination drug. Paracetamol has an antipyretic and analgesic effect. Phenylephrine reduces swelling of the mucous membranes and sinuses, resulting in easier breathing. Ascorbic acid replenishes the increased need for vitamin C during colds and flu.
Indications for use
For flu symptoms and other colds.
Contraindications
- genetic absence of glucose-6-phosphate dehydrogenase,
- diseases of the blood system,
- severe liver or kidney dysfunction,
- thyrotoxicosis,
- diabetes,
- children up to 6 years old,
- arterial hypertension,
- simultaneous use with other paracetamol-containing drugs,
- hypersensitivity to the ingredients included in the drug.
Storage conditions
Store at room temperature out of the reach of children.
special instructions
Excessive consumption of tea or coffee while taking Coldrex may cause irritability and nervous tension.
To avoid toxic liver damage, patients taking Coldrex should avoid drinking alcohol. It is not recommended to prescribe the drug for chronic alcoholism.
Use in pediatrics
children under 6 years of age without consulting a doctor .
Side effects
Individual intolerance to product components.
Use during pregnancy and breastfeeding
You can take the drug with caution and only after consulting a doctor during pregnancy and lactation.
Interaction
The drug should not be taken simultaneously with tricyclic antidepressants, beta-blockers, MAO inhibitors and within 14 days after their discontinuation.
The risk of developing hepatotoxicity increases with simultaneous use of barbiturates, diphenine, carbamazepine, rifampicin, zidovudine and other inducers of microsomal liver enzymes.
Metoclopramide and domperidone increase the rate of absorption of paracetamol, and cholestyramine reduces it.
Overdose
Symptoms: nausea, vomiting, stomach pain, sweating, pale skin, arrhythmia. After 1-2 days, symptoms of liver damage may appear. In severe cases, liver failure and coma develop. In case of overdose (even if the child feels well), the risk of delayed serious liver damage should be taken into account. Treatment: If you suspect poisoning, you should immediately consult a doctor. During the first 4 hours of poisoning, gastric lavage is indicated. A specific antidote for paracetamol poisoning is acetylcysteine.
Overdose
In case of overdose of Coldrex® Junior (even if you feel well), the risk of delayed signs of serious liver damage should be taken into account. Symptoms caused by paracetamol: within 24 hours - pale skin, loss of appetite, nausea, vomiting, abdominal pain. After 12-48 hours, signs of liver dysfunction, signs of impaired glucose metabolism and metabolic acidosis may appear. In cases of severe poisoning, severe liver failure may occur, including hepatic encephalopathy, coma and death. Acute renal failure with acute tubular necrosis, which is diagnosed by severe pain in the lumbar region, hematuria and proteinuria, can develop without severe impairment of liver function. There are reports of cases of cardiac arrhythmia and pancreatitis with an overdose of paracetamol. In the early period, symptoms may be limited only to nausea and vomiting and may not reflect the severity of the overdose or the risk of damage to internal organs. Treatment: within the first hour after the expected overdose, it is advisable to administer activated carbon orally. 4 or more hours after the suspected overdose, it is necessary to determine the concentration of paracetamol in plasma (earlier determination of paracetamol concentration may be unreliable). A specific antidote for paracetamol poisoning is acetylcysteine. Treatment with acetylcysteine can be carried out up to 24 hours after taking paracetamol, however, the maximum hepatoprotective effect can be obtained in the first 8 hours after an overdose. After this, the effectiveness of the antidote drops sharply. If necessary, acetylcysteine can be administered intravenously. In the absence of vomiting, an alternative option (if it is not possible to quickly obtain hospital care) is to prescribe methionine orally. Treatment of patients with severe liver dysfunction 24 hours after taking paracetamol should be carried out in conjunction with specialists from a toxicology center or a specialized liver disease department. Symptoms caused by phenylephrine: irritability, headache, dizziness, insomnia, increased blood pressure, nausea, vomiting, increased excitability, reflex bradycardia. In severe cases of overdose, hallucinations, confusion, convulsions, and arrhythmias may develop. Treatment: symptomatic therapy, for severe arterial hypertension, the use of alpha-blockers such as phentolamine. Symptoms caused by ascorbic acid: ascorbic acid in high doses (more than 3000 mg) can cause temporary osmotic diarrhea and gastrointestinal disturbances, such as nausea, stomach discomfort. Manifestations of an overdose of ascorbic acid can be classified as those caused by severe liver damage as a result of an overdose of paracetamol. Treatment: symptomatic, forced diuresis.
Coldrex Junior (powder)
The drug should be taken only in recommended doses!
If you suspect an overdose, even if you feel well, you must stop using the drug and consult a doctor immediately, because There is a risk of delayed serious liver damage and hospitalization may be required.
Overdose is usually caused by paracetamol.
Symptoms (due to paracetamol)
An overdose of paracetamol may cause liver failure, which may lead to the need for liver transplantation or death.
Within 24 hours it is possible: pale skin, loss of appetite, nausea, vomiting, stomach pain, sweating. Signs of liver dysfunction may appear within 12-48 hours. Signs of impaired glucose metabolism and metabolic acidosis may occur. Clinical signs of liver damage usually develop within 24-48 hours and reach a maximum after 4-6 days. In cases of severe poisoning, severe liver failure may develop, including hepatic encephalopathy, the need for a liver transplant, coma and death. Acute renal failure with acute tubular necrosis, which is diagnosed by severe pain in the lumbar region, hematuria and proteinuria, can develop without severe impairment of liver function. There are reports of cases of cardiac arrhythmia and acute pancreatitis with an overdose of paracetamol.
At the first signs of an overdose, you should immediately consult a doctor, even in the absence of clear symptoms of poisoning.
In the early period, symptoms may be limited only to nausea and vomiting and may not reflect the severity of the overdose or the risk of damage to internal organs.
Treatment:
During the first hour after the expected overdose, it is advisable to administer activated carbon orally. Four or more hours after the expected overdose, it is necessary to determine the concentration of paracetamol in the blood plasma (earlier determination of the concentration of paracetamol may be unreliable). Treatment with acetylcysteine can be carried out up to 24 hours after taking paracetamol, however, the maximum hepatoprotective effect can be obtained in the first 8 hours after an overdose. After this, the effectiveness of the antidote drops sharply. If necessary, acetylcysteine can be administered intravenously. In the absence of vomiting, an alternative option (if it is not possible to quickly obtain hospital care) is to prescribe methionine orally. Treatment of patients with severe liver dysfunction 24 hours after taking paracetamol should be carried out in conjunction with specialists from a poison control center or specialized liver disease department.
Symptoms (due to phenylephrine)
Symptoms of a phenylephrine overdose are similar to those of side effects. In addition: increased blood pressure, reflex bradycardia. In severe cases of overdose, hallucinations, confusion, convulsions, and arrhythmias may develop. It should be borne in mind that the appearance of clinically significant symptoms of phenylephrine overdose when taking the drug is always associated with severe liver damage due to an overdose of paracetamol.
Treatment:
symptomatic therapy, for severe arterial hypertension, the use of alpha-blockers such as phentolamine.
Symptoms (due to ascorbic acid)
High doses of ascorbic acid (more than 3000 mg) can cause temporary osmotic diarrhea and gastrointestinal disturbances, such as nausea and stomach discomfort. It should be borne in mind that the appearance of clinically significant symptoms of an overdose of ascorbic acid when taking the drug is always associated with severe liver damage due to an overdose of paracetamol.
Treatment:
symptomatic, forced diuresis.
Side effect
Determination of the frequency of side effects: very often (≥1/10), often (≥1/100 and <1/10), infrequently (≥1/1000 and <1/100), rarely (≥1/10,000 and <1 /1000), very rare (≥1/100,000 and <1/10,000). At recommended doses, the drug is usually well tolerated. Paracetamol rarely has side effects. With long-term use in excess of the recommended dose, hepatotoxic and nephrotoxic effects may occur. From the hematopoietic system: very rarely - thrombocytopenia. Allergic reactions: rarely - skin rash, urticaria, allergic dermatitis; very rarely - anaphylaxis, hypersensitivity reactions, incl. angioedema, Stevens-Johnson syndrome. From the respiratory system: very rarely - bronchospasm in patients sensitive to acetylsalicylic acid and other NSAIDs. From the digestive system: very rarely - nausea, vomiting, liver dysfunction. From the side of the central nervous system: very rarely - dizziness, headache, insomnia. From the sensory organs: rarely - mydriasis, acute attack of glaucoma in most cases in patients with angle-closure glaucoma. From the cardiovascular system: rarely - tachycardia, palpitations, increased blood pressure. From the urinary system: very rarely - dysuria, urinary retention in patients with obstruction of the bladder outlet due to prostatic hypertrophy. If any adverse reactions occur, the patient should consult a doctor.
Coldrex Junior
The drug should be taken only in recommended doses!
If you suspect an overdose, even if you feel well, you must stop using the drug and consult a doctor immediately, because There is a risk of delayed serious liver damage and hospitalization may be required. Overdose is usually caused by paracetamol.
Symptoms (due to paracetamol)
An overdose of paracetamol may cause liver failure, which may lead to the need for liver transplantation or death.
Within 24 hours it is possible: pale skin, loss of appetite, nausea, vomiting, stomach pain, sweating. Signs of liver dysfunction may appear within 12-48 hours. Signs of impaired glucose metabolism and metabolic acidosis may occur. Clinical signs of liver damage usually develop within 24-48 hours and reach a maximum after 4-6 days. In cases of severe poisoning, severe liver failure may develop, including hepatic encephalopathy, the need for a liver transplant, coma and death.
Acute renal failure with acute tubular necrosis, which is diagnosed by severe pain in the lumbar region, hematuria and proteinuria, can develop without severe impairment of liver function.
There are reports of cases of cardiac arrhythmia and acute pancreatitis with an overdose of paracetamol.
At the first signs of an overdose, you should immediately consult a doctor,
even in the absence of clear symptoms of poisoning
. In the early period, symptoms may be limited only to nausea and vomiting and may not reflect the severity of the overdose or the risk of damage to internal organs.
Treatment
: within the first hour after the expected overdose, it is advisable to administer activated carbon orally. Four or more hours after the expected overdose, it is necessary to determine the concentration of paracetamol in the blood plasma (earlier determination of the concentration of paracetamol may be unreliable).
Treatment with acetylcysteine can be carried out up to 24 hours after taking paracetamol, however, the maximum hepatoprotective effect can be obtained in the first 8 hours after an overdose. After this, the effectiveness of the antidote drops sharply. If necessary, acetylcysteine can be administered intravenously.
In the absence of vomiting, an alternative option (if it is not possible to quickly obtain hospital care) is to prescribe methionine orally.
Treatment of patients with severe liver dysfunction 24 hours after taking paracetamol should be carried out in conjunction with specialists from a poison control center or specialized liver disease department.
Symptoms (due to phenylephrine)
Symptoms of a phenylephrine overdose are similar to those of side effects.
In addition: increased blood pressure, reflex bradycardia. In severe cases of overdose, hallucinations, confusion, convulsions, and arrhythmias may develop. It should be borne in mind that the appearance of clinically significant symptoms of phenylephrine overdose when taking the drug is always associated with severe liver damage due to an overdose of paracetamol.
Treatment
: symptomatic therapy, for severe arterial hypertension, the use of alpha-blockers such as phentolamine.
Symptoms (due to ascorbic acid)
High doses of ascorbic acid (more than 3000 mg) can cause temporary osmotic diarrhea and gastrointestinal disturbances, such as nausea and stomach discomfort. It should be borne in mind that the appearance of clinically significant symptoms of an overdose of ascorbic acid when taking the drug is always associated with severe liver damage due to an overdose of paracetamol.
Treatment
: symptomatic, forced diuresis.
Interaction with other drugs
Paracetamol, when taken for a long time, enhances the effect of indirect anticoagulants (warfarin and other coumarins), which increases the risk of bleeding. Occasional administration of a single dose of the drug does not have a significant effect on the effect of indirect anticoagulants. Inducers of microsomal oxidation enzymes in the liver (barbiturates, diphenin, carbamazepine, rifampicin, zidovudine, phenytoin, ethanol, flumecinol, phenylbutazone and tricyclic antidepressants) increase the risk of hepatotoxicity in overdoses and concomitant use with paracetamol. Microsomal oxidation inhibitors (cimetidine) reduce the risk of hepatotoxicity. Paracetamol reduces the effectiveness of diuretics. Metoclopramide and domperidone increase, and cholestyramine reduces the rate of absorption of paracetamol. Paracetamol enhances the effects of MAO inhibitors, sedatives, ethanol. Phenylephrine when taken with MAO inhibitors can lead to an increase in blood pressure. Phenylephrine reduces the effectiveness of beta-blockers and antihypertensive drugs, increases the risk of developing arterial hypertension and disorders of the cardiovascular system. Tricyclic antidepressants enhance the sympathomimetic effect of phenylephrine and may increase the risk of side effects from the cardiovascular system. Concomitant use of halothane with phenylephrine increases the risk of developing ventricular arrhythmia. Phenylephrine reduces the hypotensive effect of guanethidine, which, in turn, enhances the alpha-adrenergic stimulating activity of phenylephrine. Antidepressants, antiparkinsonian drugs, antipsychotic drugs, phenothiazine derivatives increase the risk of developing urinary retention, dry mouth, and constipation. The simultaneous administration of GCS with phenylephrine increases the risk of developing glaucoma. When used simultaneously with digoxin and cardiac glycosides, the risk of developing heart rhythm disturbances or a heart attack may increase. Ascorbic acid, when used simultaneously with iron preparations, due to its restorative properties, converts ferric iron into divalent iron, which helps improve its absorption. When used concomitantly, ascorbic acid increases iron excretion in patients receiving deferoxamine. When used simultaneously with barbiturates and primidone, the excretion of ascorbic acid in the urine increases. Ascorbic acid in high doses can reduce urine pH, which, when used simultaneously, reduces the tubular reabsorption of amphetamine and tricyclic antidepressants. When used simultaneously, acetylsalicylic acid reduces the absorption of ascorbic acid by about a third. When used simultaneously with warfarin, the effects of warfarin may be reduced. When used simultaneously with tetracycline, the excretion of ascorbic acid in the urine increases.
Coldrex MaxGrip for colds and flu, lemon flavor, powder, 5 sachets
A country
Spain
Country of manufacture may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Description
Coldrex MaxGrip is a combined drug, the effect of which is determined by the active components included in the composition:
• Paracetamol has an analgesic and antipyretic effect. • Phenylephrine is a vasoconstrictor, relieves nasal congestion (constricts the vessels of the nasal mucosa and paranasal sinuses) and facilitates breathing. • Ascorbic acid (vitamin C) replenishes the need for vitamin C for colds and flu.
The active substances of Coldrex MaxGrip do not cause drowsiness.* * Instructions for medical use, RU P N014920/01
Compound
Active substances, mg Paracetamol 1000.00 Ascorbic acid 40.00 Phenylephrine hydrochloride 10.00 Excipients Sucrose 3725.0 Citric acid 680.0 Sodium citrate 430.0 Corn starch 200.0 Lemon flavoring 200.0 Sodium cyclamate 79.0 Sodium saccharinate 54.0 Curcumin dye (E100) 7.0 Colloidal silicon dioxide 2.1
Product description
Light yellow powder with a lemon scent.
pharmachologic effect
A combined product whose effect is determined by its constituent components.
Paracetamol is an analgesic and antipyretic. Its mechanism of action is believed to be the suppression of prostaglandin synthesis, primarily in the central nervous system. The absence of suppression of peripheral prostaglandin synthesis gives the drug significant pharmacological properties, such as the preservation of protective prostaglandins in the gastrointestinal tract. This property of paracetamol makes the drug particularly suitable for patients with a history of gastrointestinal disease (for example, patients with a history of gastrointestinal bleeding or elderly patients) or patients taking concomitant medications in which suppression of peripheral prostaglandin synthesis may be undesirable. Phenylephrine hydrochloride is a sympathomimetic agent, the action of which is aimed at stimulating adrenergic receptors (mainly α-adrenergic receptors), which leads to a reduction in swelling of the nasal mucosa and easier nasal breathing. Ascorbic acid (vitamin C) replenishes the increased need for vitamin C during colds and flu, especially in the initial stages of the disease, since vitamin C reserves in these conditions can be depleted and appetite decreases. Ascorbic acid is an electron donor (reducing and oxidizing agent), and perhaps all its biochemical and molecular properties may be due to this function. Pharmacokinetics Absorption and distribution Paracetamol is rapidly and almost completely absorbed from the gastrointestinal tract. Plasma protein binding is minimal when used at therapeutic concentrations. Phenylephrine hydrochloride is unevenly absorbed from the gastrointestinal tract. There are no data on the distribution of phenylephrine. Ascorbic acid is quickly absorbed from the gastrointestinal tract and distributed throughout the body. The connection with blood plasma proteins is 25%. Metabolism Paracetamol is metabolized primarily in the liver. Phenylephrine hydrochloride undergoes primary metabolism by monoamine oxidases in the intestine and liver. Thus, when administered orally, the bioavailability of phenylephrine is reduced. There are no data on the metabolism of ascorbic acid. Excretion Paracetamol is excreted by the kidneys in the form of metabolites, mainly glucuronide and sulfate conjugates; less than 5% of the dose taken is excreted unchanged. Phenylephrine hydrochloride is excreted almost entirely by the kidneys in the form of sulfate conjugates. When used in doses exceeding the body's needs for ascorbic acid, ascorbic acid is excreted by the kidneys in the form of metabolites.
Indications for use
Coldrex MaxGrip is used in adults as a temporary remedy to relieve cold and flu symptoms, including: - fever; - headache; - chills; - pain in joints and muscles; - pain in the sinuses and nasal congestion; - sore throat
Contraindications
Coldrex MaxGripp is not recommended for use in patients with: - hypersensitivity to paracetamol, phenylephrine, ascorbic acid (vitamin C) or any other component of the drug; - severe liver and kidney dysfunction; - arterial hypertension; — hyperfunction of the thyroid gland (including with thyrotoxicosis); - diseases of the blood system; — heart diseases (with severe stenosis of the aortic mouth, acute myocardial infarction, tachyarrhythmia); — prostatic hyperplasia; — diabetes mellitus and diseases associated with hereditary malabsorption of sugar (each sachet contains about 4 g of sucrose); - genetic absence of glucose-6-phosphate dehydrogenase; - sucrase/isomaltase deficiency, with a rare hereditary disorder such as fructose intolerance, glucose-galactose malabsorption, because the drug contains sucrose; - angle-closure glaucoma; - simultaneous use of tricyclic antidepressants, beta-blockers, monoamine oxidase inhibitors (MAOIs), including for a period of up to 14 days after their discontinuation; - simultaneous use of other paracetamol-containing drugs, decongestants, non-narcotic analgesics, non-steroidal anti-inflammatory drugs, drugs to relieve the symptoms of colds, flu and nasal congestion, drugs that regulate appetite, amphetamine-like psychostimulants, barbiturates, antiepileptic drugs, rifampicin, chloramphenicol (see. section “Interaction with other drugs”); - simultaneous intake of ethanol-containing drinks and medications; — chronic alcoholism; — pregnancy and during breastfeeding; - under 18 years of age.
Carefully
If you have one of the following diseases/conditions/risk factors, be sure to consult your doctor before taking the drug: - benign hyperbilirubinemia; - impaired liver and kidney function of mild to moderate severity; - alcoholic liver disease; - cardiovascular diseases, including high blood pressure, obliterating vascular diseases (Raynaud's syndrome); - pheochromocytoma; - presence of severe infections, because taking the drug may increase the risk of metabolic acidosis; - glutathione deficiency (in particular in extremely malnourished patients suffering from anorexia, chronic alcoholism or patients with a low body mass index); - simultaneous use of antihypertensive drugs, digoxin and cardiac glycosides, ergot alkaloids (for example, ergotamine and methysergide)
Use during pregnancy and lactation
Adequate and strictly controlled studies of the use of the drug during pregnancy and breastfeeding have not been conducted. In animal studies during late pregnancy, phenylephrine caused fetal growth restriction and stimulated early onset of labor. Phenylephrine may pass into breast milk. There are no data on the use of drugs containing phenylephrine during breastfeeding. The use of the drug during pregnancy and breastfeeding is contraindicated. If it is necessary to use the drug during lactation, breastfeeding should be stopped.
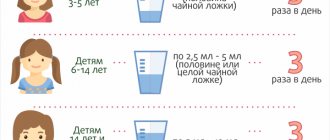
Directions for use and doses
For oral administration. Do not exceed the recommended dose! The smallest dose required to achieve the effect should be used for the shortest possible treatment period! The minimum interval between doses of Coldrex MaxGrip should be 4 hours. Pour the contents of 1 sachet into a mug, pour half a mug of hot water and mix thoroughly. Add cold water and sugar if necessary. Adults: 1 sachet every 4–6 hours, but not more than 4 sachets per day. The maximum daily dose should not exceed 4 sachets. The maximum duration of use of the drug without consulting a doctor is no more than 5 days. Do not take simultaneously with other paracetamol-containing products, decongestants and cold and flu relief products, as well as ethanol-containing products and drinks. If symptoms of the disease persist while taking the drug, you should consult a doctor.
Side effect
At recommended doses, the drug is usually well tolerated. The following adverse reactions were detected spontaneously during post-registration use of the drug. Adverse reactions are classified according to body systems and according to the frequency of development. The frequency of adverse reactions is determined as follows: often (≥ 1/100 and Paracetamol Paracetamol rarely has side effects. Blood and lymphatic system disorders Very rare: thrombocytopenia, leukopenia, agranulocytosis. Immune system disorders Very rare: anaphylactic shock, skin hypersensitivity reactions including, but not limited to, skin rash, urticaria, angioedema (Angioedema), Stevens-Johnson syndrome, toxic epidermal necrolysis Respiratory, thoracic and mediastinal disorders Very rare: bronchospasm in patients with hypersensitivity to acetylsalicylic acid and other non-steroidal anti-inflammatory drugs.Disorders of the liver and biliary tract. Very rarely: disorders of the liver.Disorders of the kidneys and urinary tract. With long-term use of the drug in doses exceeding the recommended, the likelihood of nephrotoxicity increases. Phenylephrine Immune system disorders Rare: hypersensitivity reactions, urticaria, allergic dermatitis. Mental disorders Common: nervousness. Nervous system disorders Common: headache, dizziness, insomnia. Visual disorders Rare: mydriasis, acute attack of glaucoma, in most cases in patients with angle-closure glaucoma. Cardiovascular system disorders Common: increased blood pressure. Rarely: tachycardia, palpitations. Gastrointestinal disorders Common: nausea, vomiting. Skin and subcutaneous tissue disorders Very rare: rash. Renal and urinary tract disorders Rare: dysuria, urinary retention in patients with bladder outlet obstruction due to prostatic hypertrophy. Ascorbic acid Blood and lymphatic system disorders Frequency unknown: thrombocytosis, hyperprothrombinemia, erythropenia, neutrophilic leukocytosis, hypokalemia. Immune system disorders Not known: allergic reactions (skin rash, skin flushing). Gastrointestinal disorders Not known: irritation of the gastrointestinal mucosa. When taking ascorbic acid more than 600 mg/day, moderate pollakiuria is possible. If any of the listed adverse reactions occur, stop taking the drug immediately and consult a doctor as soon as possible. If any of the adverse reactions indicated in the instructions worsen or you notice other adverse reactions not listed in the instructions, notify your doctor.
Overdose
The drug should be taken only in recommended doses! If you suspect an overdose, even if you feel well, you must stop using the drug and consult a doctor immediately, because There is a risk of delayed serious liver damage and hospitalization may be required. Overdose is usually caused by paracetamol. Paracetamol Symptoms An overdose of paracetamol may cause liver failure, which may lead to the need for a liver transplant or death. Within 24 hours the following are possible: pale skin, loss of appetite, nausea, vomiting, abdominal pain. Clinical signs of liver damage usually develop after 24–48 hours and reach a maximum after 4–6 days. Signs of impaired glucose metabolism and metabolic acidosis may occur. Toxic effects in adults are possible after taking more than 10 g of paracetamol. Acute pancreatitis has been observed, usually with liver dysfunction and liver toxicity. Acute renal failure with acute tubular necrosis, which is diagnosed by severe pain in the lumbar region, hematuria and proteinuria, can develop without severe impairment of liver function. There are reports of cases of cardiac arrhythmia with an overdose of paracetamol. Taking 5 g or more of paracetamol can lead to liver damage in patients with the following risk factors: - long-term treatment with carbamazepine, phenobarbital, phenytoin, primidone, rifampicin, St. John's wort, or other drugs that stimulate liver enzymes; - regular consumption of alcohol in excess quantities; - glutathione deficiency (due to malnutrition, cystic fibrosis, HIV infection, starvation, exhaustion). At the first signs of an overdose, you should immediately consult a doctor, even in the absence of clear symptoms of poisoning. In the early period, symptoms may be limited only to nausea and vomiting and may not reflect the severity of the overdose or the risk of damage to internal organs. Treatment During the first hour after a suspected overdose, it is advisable to administer activated carbon orally. Four or more hours after the suspected overdose, it is necessary to determine the concentration of paracetamol in the blood plasma (earlier determination of the concentration of paracetamol may be unreliable). Treatment with acetylcysteine can be carried out up to 24 hours after taking paracetamol, however, the maximum hepatoprotective effect can be obtained in the first 8 hours after an overdose. After this, the effectiveness of the antidote drops sharply. If necessary, acetylcysteine can be administered intravenously. In the absence of vomiting, an alternative option (if it is not possible to quickly obtain hospital care) is to prescribe methionine orally. Treatment of patients with severe liver dysfunction 24 hours after taking paracetamol should be carried out in conjunction with specialists from a poison control center or specialized liver disease department. Phenylephrine Symptoms Symptoms of phenylephrine overdose are similar to those of side effects. In addition: irritability, headache, dizziness, insomnia, increased excitability, increased blood pressure, nausea, vomiting, reflex bradycardia. In severe cases of overdose, hallucinations, confusion, convulsions, and arrhythmias may develop. It should be borne in mind that the appearance of clinically significant symptoms of phenylephrine overdose when taking the drug is always associated with severe liver damage due to an overdose of paracetamol. Treatment Symptomatic therapy, for severe arterial hypertension, the use of alpha-blockers, such as phentolamine. Ascorbic acid Symptoms High doses of ascorbic acid (more than 3000 mg) can cause temporary osmotic diarrhea and gastrointestinal disturbances, such as nausea, stomach discomfort. It should be borne in mind that the appearance of clinically significant symptoms of an overdose of ascorbic acid when taking the drug is always associated with severe liver damage due to an overdose of paracetamol. Treatment: Symptomatic, forced diuresis.
Interaction with other drugs
Potentially clinically significant drug interactions are presented below (see sections “Contraindications” and “Special Instructions”). Paracetamol, when taken regularly over a long period of time, enhances the effect of warfarin and other coumarins, which increases the risk of bleeding. Occasional administration of a single dose of the drug does not have a significant effect on their effect. Inducers of microsomal oxidation enzymes in the liver (barbiturates, diphenin, carbamazepine, rifampicin, zidovudine, phenytoin, ethanol, flumecinol, phenylbutazone and tricyclic antidepressants) increase the risk of hepatotoxicity in overdoses and concomitant use with paracetamol. Microsomal oxidation inhibitors (cimetidine) reduce the risk of hepatotoxicity. Paracetamol reduces the effectiveness of diuretics. Metoclopramide and domperidone increase, and cholestyramine reduces the rate of absorption of paracetamol. Paracetamol enhances the effects of MAO inhibitors, sedatives, ethanol. The simultaneous use of paracetamol and alcoholic beverages increases the risk of developing liver damage and acute pancreatitis. Phenylephrine when taken with MAO inhibitors can lead to increased blood pressure. Phenylephrine may reduce the effectiveness of beta-blockers and other antihypertensive drugs (including drugs such as debrisoquine, guanethidine, reserpine, methyldopa), and increases the risk of developing hypertension and cardiovascular disorders. Tricyclic antidepressants enhance the sympathomimetic effect of phenylephrine and may increase the risk of cardiovascular side effects. Concomitant use of halothane with phenylephrine increases the risk of developing ventricular arrhythmia. Phenylephrine reduces the hypotensive effect of guanethidine, which, in turn, enhances the alpha-adrenergic stimulating activity of phenylephrine. Antidepressants, antiparkinsonian drugs, antipsychotic drugs, phenothiazine derivatives increase the risk of developing urinary retention, dry mouth, and constipation. Concomitant use of glucocorticosteroids with phenylephrine increases the risk of developing glaucoma. Concomitant use of digoxin and other cardiac glycosides may increase the risk of heart rhythm disturbances and heart attack. Concomitant use of phenylephrine and ergot alkaloids (eg, ergotamine and methysergide) may increase the risk of ergotism. Concomitant use of phenylephrine with sympathomimetic amines may increase the risk of adverse cardiovascular effects. Ascorbic acid increases the risk of developing crystalluria during treatment with salicylates and short-acting sulfonamides, slows down the excretion of acids by the kidneys, increases the excretion of drugs that have an alkaline reaction (including alkaloids), and reduces the concentration of oral contraceptives in the blood. Ethanol contributes to the development of acute pancreatitis. Myelotoxic drugs enhance the hematotoxicity of the drug.
special instructions
You should consult your doctor if symptoms worsen, persist for more than 5 days, or if you have a high fever, skin rash, or persistent headache. The drug should not be taken simultaneously with other paracetamol-containing drugs, as well as non-narcotic analgesics, NSAIDs (metamizole sodium, acetylsalicylic acid, ibuprofen, etc.), drugs to eliminate the symptoms of colds and flu, sympathomimetics (decongestants), drugs that regulate appetite , amphetamine-like psychostimulants, barbiturates, antiepileptic drugs, rifampicin, chloramphenicol. To avoid toxic liver damage, the drug should not be combined with ethanol-containing drugs and drinks, or taken by people prone to chronic alcohol consumption. Concomitant liver disease increases the risk of further liver damage while taking the drug. When taking the drug in patients with non-alcoholic cirrhosis of the liver, there is a high risk of overdose. When performing tests to determine uric acid and blood glucose levels, tell your doctor about the use of Coldrex MaxGrip, as the drug may distort the results of laboratory tests that assess the concentration of glucose and uric acid. Patients with glutathione deficiency due to an eating disorder, cystic fibrosis, HIV infection, starvation, malnutrition are susceptible to overdose, so precautions must be taken and it is recommended to consult a doctor before taking the drug. Cases of liver failure/impaired liver function have been reported with a small overdose of paracetamol (5 g or more) in patients with low glutathione levels, in particular in extremely malnourished patients suffering from anorexia, chronic alcoholism, patients with a low body mass index or sepsis. Use of the drug in patients with low levels of glutathione, for example, with sepsis, may increase the risk of developing metabolic acidosis, accompanied by symptoms of rapid, difficult breathing (feeling short of breath, shortness of breath), nausea, vomiting, loss of appetite. If these symptoms occur simultaneously, you should immediately consult a doctor. Before taking Coldrex MaxGrip, you should consult your doctor if you are taking: - warfarin or other indirect anticoagulants to thin the blood; - metoclopramide, domperidone (used to relieve nausea and vomiting) or cholestyramine, used to lower blood cholesterol. Each sachet contains 0.12 g sodium, this should be taken into account in patients on a hyposodium diet
Release form
Powder for preparing a solution for oral administration (lemon). 6.427 g of powder in multi-layer bags (paper/aluminum foil/polyethylene). 5 sachets along with instructions for use are placed in a cardboard box. Secondary packaging is allowed to have a first-opening control.
Storage conditions
Store at a temperature not exceeding 25 C. Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date stated on the package.