Release form and composition
The dosage form of Maruxa is film-coated tablets: white, oval, biconvex; 10 mg tablets – on one side with a score; on the fracture there is a white, uneven, rough surface (in a cardboard pack there are 3 or 6 strip packs of 10 tablets each).
Composition of 1 tablet:
- active substance: memantine hydrochloride – 10 or 20 mg;
- auxiliary components (10/20 mg): colloidal silicon dioxide – 2.5/5 mg; Magnesium stearate – 1.25/2.5 mg; lactose monohydrate – 51.45/102.9 mg; talc – 9.8/19.6 mg; microcrystalline cellulose – 175/350 mg;
- shell (10/20 mg): simethicone – 0.01/0.02 mg; 30% aqueous dispersion of copolymer of ethyl acrylate and methacrylic acid (1:1) – 0.6/1.2 mg; triacetin – 0.12/0.24 mg; talc – 0.27/0.54 mg.
Maruxa instructions
Maruxa instructions for use Maruxa tablets 10 mg No. 30**
Maruxa tablets Maruxa
Composition of Marux
Dosage form
: film-coated tablets
Composition for 1 tablet
Core:
Active substance:
Memantine hydrochloride 10.00 mg
Excipients:
51.45 mg, microcrystalline cellulose 175.00 mg, colloidal silicon dioxide 2.50 mg, talc 9.80 mg, magnesium stearate 1.25 mg
Film shell: methacrylic acid and ethyl acrylate copolymer (1: 1), 30% aqueous dispersion 0.60 mg, talc 0.27 mg, triacetin 0.12 mg, simethicone 0.01 mg 30% aqueous dispersion contains, in addition to methacrylic acid and ethyl acrylate copolymer, also sodium lauryl sulfate (0.7% calculated on dry matter in suspension) and polysorbate-80 (2.3% calculated on dry matter in suspension) as emulsifiers.
Expressed as the mass of dry matter included in a 30% aqueous dispersion.
Pharmacological properties
Pharmacodynamics
Memantine is an adamantane derivative, a non-competitive antagonist of N-methyl-D-aspartate (NMDA) receptors. It has a modulating effect on the glutamatergic system.
Main properties of memantine:
- blocking calcium channels;
- regulation of ion transport;
- improving the process of nerve impulse transmission;
- normalization of membrane potential;
- increased daily activity;
- improvement of cognitive processes.
Pharmacokinetics
Memantine is quickly and completely absorbed after taking Maruxa orally. Tmax (time to reach maximum concentration) in blood plasma ranges from 3 to 8 hours. In the absence of disturbances in renal function, accumulation of the substance is not observed.
When taking Maruxa daily at a daily dose of 20 mg, the Css (equilibrium concentration) of the substance in the blood plasma is 70–150 ng/ml. When taking the drug in a daily dose of 5–30 mg, the calculated ratio of the average concentration in the cerebrospinal fluid to the plasma concentration is 0.52. Vd (volume of distribution) – approximately 10 l/kg. Plasma protein binding is approximately 45%.
Excretion occurs mainly unchanged (about 80%). The main metabolites are N-3,5-dimethylgludantan, 1-nitroso-3-5-dimethyladamantane and an isomeric mixture of 4- and 6-hydroxy-memantine (they do not have their own pharmacological activity). Metabolism carried out by cytochrome P450 isoenzymes has not been identified. On average, 84% of the dose is excreted over 20 days, predominantly (more than 99%) by the kidneys.
It is excreted from the body monoexponentially. T1/2 (half-life) of the terminal phase is 60–100 hours. Total clearance in the absence of renal dysfunction is 170 ml/min/1.73 m2, part of the total renal clearance is achieved through tubular secretion. Renal excretion of memantine also involves tubular reabsorption, which may be indirectly related to cationic transport proteins. Under conditions of alkaline urine reaction, the rate of renal elimination of memantine can decrease by 7–9 times. Alkalinization of urine can be associated with a sudden change in diet, for example, when switching from a diet that includes animal products to a vegetarian diet, or as a result of intensive use of alkaline gastric buffers.
The linearity of the pharmacokinetic parameters of memantine has been proven for the dose range of 10–40 mg per day.
The level of concentration in the cerebrospinal fluid when using memantine at a dose of 20 mg per day corresponds to the value of the inhibition constant (0.5 μmol in the area of the frontal cortex of the brain).
Experience of many years of using Akatinol for Alzheimer's disease
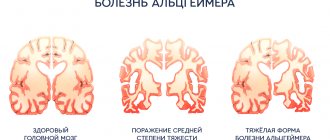
The progressive dementia seen in Alzheimer's disease has long been considered fatal because it is associated with the death of brain cells and subsequent brain atrophy. The last decade has forced us to reconsider these seemingly unshakable provisions (1). In the group of new ones, the so-called. Akatinol was identified as an “antidementia” drug. The results of its successful clinical trial in our country were published back in 1995 (2). Subsequently, it turned out that this drug is effective not only for mild, but also for moderately severe and severe types of dementia. It turned out that with its use, even at the stage of severe dementia, it is possible to compensate for neurodegenerative destruction due to the activation of glumatergic neurons and synapses with preserved NMDA receptors that have not yet died, but are in a state of apoptosis (functional shutdown, programmed cell death).
However, to achieve a therapeutic result, therapy with Akatinol must be long-term (at least six months) and continuous (3). To date, Akatinol has become one of the popular means of medical practice in the treatment of dementia. But as the scope of its use expands, questions arise regarding the limits of the duration of treatment with this drug (limited to certain courses or for life), regimens and dosages of maintenance therapy, and its tolerability by older people when taken for many years. Traditional clinical trials cannot answer these questions. There are no special studies yet on the effectiveness of extremely long-term (many years) and maintenance therapy for dementia. In these conditions, you have to turn to medical experience.
Quite early (since 1996) we began treating patients with dementia due to Alzheimer's disease and other brain pathologies (usually vascular) with Akatinol. Akatinol is very popular in psychiatric institutions in the Moscow region. Experience of its long-term use is gradually accumulating.
In this regard, the case of treatment of severe dementia as a result of Alzheimer's disease, which we have been observing since the summer of 1999 (for 11 years), is noteworthy. This case is especially interesting in that continuous and long-term use of Akatinol for 5-6 months of therapy led to a “dramatic” exit from typical Alzheimer’s type dementia to a state of practical recovery. This case was presented at the “Man and Medicine” congress in Moscow in 2000 (4).
Let us remember that we were talking about a woman born in 1924 (she is now 86 years old), who has been observed at the regional psychoneurological clinic since April 1999. Here is a brief history of her illness.
Anamnesis.
Ill since 1997 (since 74 years old). The disease developed gradually and progressed slowly. Its first manifestation was difficulties in writing. I first noticed this when I received my pension from the savings bank, when I suddenly couldn’t reproduce my signature, although I had done it easily before. Now her painting turned out different every time, and it was necessary to repeat it many times. Then her husband noticed that she stopped reading books and solving crossword puzzles, although she had previously been very keen on these activities. Then I lost interest in watching TV. Physical weakness began to gradually increase. There was uncertainty when walking, coordination of movements was impaired to such an extent that at times she had to be supported so that she did not fall. She actually fell several times, then got up on her own. She didn’t show any concern about this. Irritability appeared, which was not typical for her before. Memory noticeably weakened. She began to speak little and quietly. Sometimes my husband had the feeling that she was no longer understanding him: he asked her about one thing, and in response she said something unrelated to the question. All this grew slowly over two years, from the end of 1997 to 1999. Neither the patient nor her husband paid serious attention to these disorders, since they considered them manifestations of age.
In January 1999 (at the age of 75) she began to complain of pain in the heart area, palpitations, interruptions in heart function intensified once again, shortness of breath and swelling of the lower extremities appeared. I contacted a therapist at the district clinic. The ECG revealed atrial fibrillation. A diagnosis was made: “ischemic arrhythmia”. Treatment was carried out on an outpatient basis (finoptin, veroshpiron, furosemide, aspirin). The somatic condition returned to normal relatively quickly. However, after this, his mental state began to deteriorate. It started with difficulties in orientation in space, which intensified. I began to get confused even in my own apartment. Gradually I completely lost my bearings: I couldn’t find my bed or toilet. Memory has deteriorated sharply. I even began to forget my husband’s name. Then she stopped recognizing her own granddaughter and asked who she was; I didn’t recognize my husband either. Sometimes she took him for her father, but more often she considered him just a stranger. She began to claim that she lives in Dnepropetrovsk, where she actually lived many years ago. She began to speak very little, quietly, in short, monosyllabic phrases. At times she became aggressive and complained that they wanted to rob her. She claimed that some women and men were entering the apartment and allegedly beating her. In this state, on the advice of a neurologist, in April 1999 she first turned to a psychiatrist at the regional psychoneurological clinic (her husband took her there). Mental status (according to the outpatient card): “Moves with difficulty, with the help of her husband. Contact is difficult. With difficulty, only after repeating it twice, she understands the questions addressed to her. Emotionally depressed. Lethargic, apathetic. Intellectually and mnestically, the ability to write is sharply reduced. According to the husband, he occasionally experiences auditory hallucinations, especially at night, and expresses ideas of persecution.”
During an examination at home in April 1999: “He has no sense of time. He cannot name not only the current day or month, but also the current year and even the season of the year. Looking out the window, he says that it is winter now. Cannot give his passport details, age, year of birth. Doesn't recognize her husband. He believes that he is with his relatives, but he does not know where. He doesn’t know his way around the apartment, doesn’t know how to get to the toilet or to his room. It is difficult to attract attention. The facial expression is unclear, the look is confused. Accounting operations are difficult. Unable to perform sequential subtraction. Tries to read, but cannot reproduce what he read. He takes dictation, but what is written can be understood with difficulty. He misses letters in a letter, distorts the style of letters, superimposes the spelling of some letters onto others, and does not complete some words. Of the several objects shown to her, she could name only two (a watch and a pen). He reacts to his inadequacy with a helpless smile. Elementary praxis (the ability to dress, wash, eat) is still preserved.”
In May 1999, a computed tomography scan of the brain was performed. A symmetrical proportional expansion of the lateral ventricles, cisterns, Sylvian fissures, the third ventricle, and, to a lesser extent, the convexity grooves of both cerebral hemispheres was revealed. Periventricular symmetrical zones of low density without mass effect. The midline structures are not displaced. The fourth ventricle is not dilated. The skull bones are not deformed. Conclusion: “Atrophic hydrocephalus of mixed type is above average, with a predominance of internal; signs of an intracranial space-occupying process were not identified. After the examination, the patient was diagnosed with dementia due to Alzheimer’s disease, and disability group I was established.”
The patient began taking Akatinol in mid-April 1999 according to the standard regimen. At first there was no change in her condition. The disease continued to progress: she lost her remaining self-care skills and could not dress or wash herself. However, subsequently (after about a month of therapy) further deterioration stopped. Then the first signs of improvement appeared in the form of general calmness, a decrease in nighttime agitation and getting ready for the trip. After 5 months of continuous use of the drug (August 1999), the husband was surprised to notice that the patient began to independently find her way to the toilet and generally began to navigate better in the apartment. Then she suddenly became interested in TV, began watching TV shows, at first everything in a row, then she began to look for a TV program and choose what to watch. At the end of September, she suddenly sat down in the kitchen and, as she loved before, began to play solitaire. By the beginning of 2000, my memory and orientation were almost completely restored, and I had a desire to communicate with old friends by phone. All subsequent time, the patient’s condition and behavior did not differ from what it was before the illness. In the spring of 2000, she underwent successful cataract surgery, after which she began to read a lot again. She spent the summer at the dacha, often remained there alone, without her husband, and dealt with all household chores on her own. She continued to take Akatinol at a daily dose of 30 mg (15 mg 2 times a day). In March 2001, due to interruptions in the supply of the drug, the daily dose was reduced to 10 mg, and soon she felt the onset of absent-mindedness, difficulty concentrating, and weakening of memory. I increased the dose to 20 mg per day, after which all the emerging disorders disappeared.
In April 2001, the patient underwent a control clinical study. The somatic condition is satisfactory, there were no signs of coronary heart disease or vascular damage to the brain, and she did not contact a therapist or neurologist. Mental status: “Accurately oriented in time.” Understands where she is and who surrounds her, the purpose of the examination and conversation with her, willingly and with interest participates in the conversation, gives complete and adequate answers to questions. The mood is even. Names the objects presented to her correctly. Sequential subtraction by 7 is quick and error-free. Accurately follows all instructions during the examination. Reading and writing are not impaired. She understands that she suffered some kind of severe mental disorder, but she cannot talk about its manifestations in herself, since it does not remain in her memory. He remembers nothing about meetings with the doctor, about the examinations performed, or about his behavior at home during that period. She knows about what happened to her only from the words of her husband. I am happy that I got rid of this serious illness and live a full, satisfying life. During an experimental psychological study, it was noted that the patient correctly understands the purpose of the study, demonstrates sufficient productivity of mental performance, an average rate of sensorimotor reactions according to the Schulte table, but is easily tired. Her self-esteem is unstable, and on the “happiness” scale she is sharply overestimated (“at my age I am the happiest”). Intellectual-mnestic activity reveals a slight decrease in intellectual capabilities, slowness and difficulty of intellectual processes and counting operations. The learning curve for 10 words is sufficient (7, 8, 8, 9, 8, 8), after an hour (delayed reproduction) - 6 words. When ten words are reproduced, extra words appear, which may indicate slight depletion of the mnestic function. Semantic memorization (mediation according to Leontiev, IV series) is available: out of 9 mediated words, he accurately names 7, approximately - 1, does not remember - 1. Computed tomography was repeated - in the same institution and on the same apparatus as the first tomographic study in May 1999. Conclusion: “In a series of control axial tomograms in comparison with X-ray CT data from May 18, 1999, the CT picture is without significant dynamics. Manifestations of high-grade mixed atrophic hydrocephalus persist.”
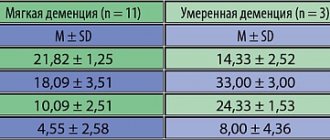
For the next 5 years, the patient led an active lifestyle. For the first two or three years, she spent the summer at the dacha, often remained there alone and successfully coped with all the chores around the house and caring for the plants. Then, by the age of 82, she gradually moved away from the dacha worries, which had become unbearable for her. Living in a city apartment, she led a normal, familiar lifestyle - she watched TV shows, read a lot, talked with friends and family. There were practically no complaints about my health; I didn’t go to the clinic. In 2004, she gradually reduced the dose of Akatinol to 10 mg per day. During this period, she experienced the sudden death of her husband, who earlier, when she was in a state of dementia, provided constant care for her and provided her with early, and most importantly, continuous and long-term use of Akatinol. She took her husband’s death hard, but without any health consequences. The medicine (Akatinol) continued to be taken at the same dose (10 mg per day). At first, the city health department allowed the patient to be provided with the drug at the expense of municipal funds, but then this was denied to her. Financial difficulties arose. Her family helped her regularly buy the drug, but for reasons of economy, she began to try to take breaks in treatment or reduce the dose. It turned out that already a week after the break in taking the drug, her condition worsened: difficulties appeared in understanding her surroundings, which was especially noticeable when trying to fill out receipts for utility bills (she could not calculate how much to pay for light). In her head, as she herself later said, “some kind of mess” arose. These phenomena disappeared soon after resuming Akatinol intake or increasing its dose. So, empirically, experimentally, she herself determined that the lower limit of the daily daily dose of Akatinol for her is 5 mg (half a tablet). As soon as she switched to taking ¼ tablet (2.5 mg), problems with comprehension, memory and counting immediately appeared. I still regularly take ½ tablet daily. (5 mg) Akatinol. The condition remains normal for her. Lives with his grandson, who very often goes on business trips for several days. She runs the household independently, prepares food for him and for herself. Copes with all these tasks without problems. She willingly agreed to go to the clinic for a follow-up examination. On the way, she discovered excellent navigation skills - in the intricate labyrinths of blocks of standard five-story buildings, where the young driver got confused, she, like a “navigator,” led him to the right road.
Clinical and psychological research was carried out at the Clinic of Nervous Diseases named after. AND I. Kozhevnikov (I.M. Sechenov Medical Academy) in February 2006. The examination was conducted by Doctor of Medical Sciences V.V. Zakharov. Here are his results: “When examined in clear consciousness, contact, adequate, correctly oriented in place and time. Moderate cognitive impairment was noted (MMSE 25 points). Neuropsychological testing reveals moderate impairment of executive functions in the form of decreased speech fluency and decreased concentration. Memory is impaired to a small extent, mainly of the dynamic type. Speech, praxis, gnosis - without visible disturbances. The neurological status shows revival of oral automatism reflexes, hypomimia, and hypokinesia. There are no paresis. Tendon reflexes are lively, uniform, and there are no pathological ones. Sensitivity is intact. Coordinator tests are performed satisfactorily. She is stable in the Romberg position. Gait is without any peculiarities. Controls the pelvic organs. An MRI examination of the head was performed. The resulting images revealed that the midline structures of the brain were not displaced. The lateral ventricles are symmetrically dilated. The subarachnoid spaces of the cerebral hemispheres are expanded in the temporoparietal regions. The cerebrospinal junction is without visible changes, the lower edge of the cerebellar tonsils is located at the level of Chamberlain's line. The structure of the spinal cord parenchyma at the level of C1-3 vertebrae is homogeneous. No additional formations were identified in the spinal canal at the upper cervical level. To this day (May 2010) she is alive and active, although physically weakened. Recently I called and introduced myself: “This is the exhibit that you showed to the doctors.” I inquired about the possibilities of obtaining Akatinol at preferential rates. She still takes it at her established maintenance dose (5 mg per day). There are no intellectual problems."
This incident turned out to be instructive for us in many ways. In addition to the fact that the amazing effectiveness of Akatinol in fairly severe dementia was obvious, we could see that the elimination of symptoms of dementia is not associated with the restoration of dead brain tissue: the pattern of atrophy of the cerebral cortex and subcortical structures remained stable during therapy. But the process of neurodestruction was stopped, atrophic phenomena no longer increased. Hence the obvious conclusion: the neuroprotective effect is associated with the functional restoration of neurons, with their removal from the state of apoptosis. We believe that a certain role in this was played by the fact that treatment was started relatively early, two months after the onset of the clinical picture of dementia itself.
It became clear that dementia therapy could not be stopped. Akatinol, with all its wonderful medicinal properties, is not an etiotropic drug in the treatment of Alzheimer's disease, but rather a pathogenetic drug. While normalizing one of the important links in the pathogenesis of the disease, it does not eliminate the disease itself and therefore must be used for life.
It turned out to be very important that long-term therapy with Akatinol for Alzheimer’s disease can be carried out according to the model of maintenance therapy for chronic diseases, and to maintain the therapeutic effect, the doses of the drug with which treatment began are not needed at all - but not below a certain minimum. For this patient, the ratio of therapeutic (20 mg) and maintenance (5 mg) doses was 1:4. Perhaps this rule is of a general nature and can be applied to other patients - this should be confirmed by further experience. But the very fact that the therapeutic and maintenance doses are not equal is very important, since this circumstance significantly reduces the cost of treatment. In modern conditions, this purely economic factor is often a decisive condition for success.
Other observations in which we were also able to see a very significant reverse trend in the symptoms of dementia taught us how important it is to maintain continuity of therapy in severe dementia. Thus, in one of the hospitals in the Moscow region, a patient was discharged in good condition, with restoration of her intellectual status, and she was recommended to continue treatment at home. But the patient’s husband thought that she was already good, there was no money to purchase a sufficiently expensive drug, and she was left without treatment. Two months later she was again admitted to hospital with severe dementia. Repeated therapy with Akatinol was unsuccessful - obviously, the resource for functional restoration of brain activity in Alzheimer's disease is very small and must be protected.
As for moderate and especially mild forms of dementia, here, according to our observations, intermittent therapy is also possible - for example, 2-3 month courses with a break of 3-4 weeks. However, experience shows that rigid regimens in the treatment of Alzheimer's disease are inappropriate. Everything here is individual, and that is why it is so important to establish an atmosphere of therapeutic cooperation with the patient (and his family). The patient, as was shown in the above case, is himself able to notice the beginning of intellectual deterioration (“mess in the head”) and understand that this is a signal for the immediate resumption of therapy.
Long-term therapy with Akatinol is safe; we encountered virtually no undesirable effects during its implementation.
Contraindications
Absolute:
- lactase deficiency, lactose intolerance, glucose-galactose malabsorption syndrome;
- severe liver failure (Child-Pugh scale, class C);
- age under 18 years;
- pregnancy and lactation;
- individual intolerance to the components of the drug.
Relative (Maruxa is prescribed under medical supervision):
- renal tubular acidosis;
- factors that increase urine pH (sudden changes in diet, for example, switching to a vegetarian diet, copious intake of alkaline gastric buffers);
- severe urinary tract infections caused by Proteus spp.;
- aggravated anamnesis of myocardial infarction;
- heart failure (according to the NYHA classification – functional class III–IV);
- epilepsy;
- predisposition to the development of seizures;
- thyrotoxicosis;
- combined use with other NMDA receptor antagonists (ketamine, amantadine, dextromethorphan);
- renal/liver failure;
- uncontrolled arterial hypertension.
Pregnancy and breastfeeding period
- Age up to 18 years (efficacy and safety have not been established).
- Lactase deficiency, lactose intolerance, glucose-galactose malabsorption syndrome, because Maruxa® contains lactose.
With caution: epilepsy, thyrotoxicosis, predisposition to the development of seizures; simultaneous use of NMDA receptor antagonists (amantadine, ketamine, dextromethorphan), factors that increase urine pH (sudden change of diet, for example, switching to vegetarianism, copious intake of alkaline gastric buffers), renal tubular acidosis, severe urinary tract infections caused by Proteus spp. , myocardial infarction (history), heart failure of functional class III-IV according to the NYHA classification, uncontrolled arterial hypertension, renal failure, liver failure.
Use during pregnancy and breastfeeding
Due to possible intrauterine growth retardation, Maruxa® is not used during pregnancy.
There is no information about the excretion of memantine in breast milk. However, given the lipophilicity of memantine, isolation is possible. Therefore, breastfeeding should be stopped during treatment with Maruxa®.
Instructions for use of Maruxa: method and dosage
Maruxa is taken orally, regardless of food intake. The drug should always be taken at the same time, once a day.
Therapy can only be started if the patient's caregiver regularly monitors the medication intake. The drug should be used under the supervision of a physician experienced in the diagnosis and treatment of dementia due to Alzheimer's disease.
During the first three months of treatment, the tolerability and dose of Maruxa should be regularly assessed. In the future, the clinical effectiveness of the drug and tolerability of therapy should be assessed. If well tolerated and there is a therapeutic effect, Maruxa can be taken indefinitely.
A gradual increase in the daily dose helps reduce the likelihood of side effects. The initial daily dose is 5 mg. Then, during the first three weeks, it is increased once a week to 10, 15 and 20 (recommended maintenance dose) mg.
Recommended use of Maruxa for renal dysfunction (depending on creatinine clearance):
- 30–49 ml/min: 10 mg per day; if well tolerated over 7 days, the dose according to the standard regimen can be increased to 20 mg;
- 5–29 ml/min: 10 mg per day.
Directions for use and doses
Therapy should be supervised by a physician experienced in diagnosing and treating Alzheimer's dementia. Therapy should only be started if the patient's caregiver regularly monitors the patient's medication intake. The diagnosis should be made in accordance with current guidelines.
The tolerability and dosage of Maruxa® should be regularly assessed, preferably within three months after initiation of therapy. The clinical efficacy of the drug and tolerability of therapy should then be regularly assessed in accordance with current clinical guidelines. Maintenance therapy can be continued indefinitely if there is a therapeutic effect and Maruxa® is well tolerated. The use of Maruxa should be discontinued if the therapeutic effect is no longer observed or if the patient cannot tolerate the therapy.
Inside, once a day and always at the same time, regardless of meals.
In order to reduce the risk of side effects, a gradual increase in dose is recommended: 5 mg per week during the first 3 weeks of therapy. The recommended maintenance dose is 20 mg per day.
The following dosage regimen is recommended:
1st week (1-7 days): daily dose - 5 mg (1A Marux® 10 mg tablets every day for 7 days);
2nd week (8-14 days): daily dose - 10 mg (1 tablet of Marux® 10 mg every day for 7 days);
3rd week (15-21 days): daily dose - 15 mg (according to VA Marux® tablets 10 mg every day for 7 days);
Starting from the 4th week: daily dose - 20 mg (2 Marux® 10 mg tablets every day). Elderly patients (over 65 years old)
No dose adjustment is required.
Renal dysfunction
In patients with a creatinine clearance (CC) of 50-80 ml/min, no dose adjustment is required. For patients with moderate renal failure (creatinine clearance 3049 ml/min) it is recommended
10 mg/day. If the drug is well tolerated within 7 days, the dose can be increased to 20 mg/day according to the standard regimen. In patients with severe renal failure (creatinine clearance 5-29 ml/min), the daily dose should be 10 mg/day.
Liver dysfunction
In patients with mild to moderate liver dysfunction (class A and B on the Child'Pugh scale), no dose adjustment is required.
Side effects
The severity of adverse reactions when taking Maruxa is usually mild to moderate in severity. The most common symptoms are dizziness, headache, constipation, hypertension and drowsiness.
Possible adverse reactions (> 10% - very common; > 1% and < 10% - often; > 0.1% and < 1% - uncommon; > 0.01% and < 0.1% - rare; < 0. 01% – very rarely; with uncertain frequency – in cases where the frequency of occurrence of the disorder cannot be determined from the available information):
- lymphatic system and blood: with unknown frequency - thrombocytopenic purpura, leukopenia (including neutropenia), thrombocytopenia, pancytopenia, agranulocytosis;
- parasitic/infectious diseases: rarely – fungal infections;
- immune system: often – hypersensitivity reactions;
- nervous system: often – imbalance, dizziness; infrequently - gait disturbance; very rarely - convulsions;
- psyche: often – drowsiness; infrequently – hallucinations, confusion; with uncertain frequency – psychotic reactions;
- respiratory system: often – shortness of breath;
- cardiovascular system: often - increased blood pressure; uncommon – venous thrombosis/thromboembolism, heart failure;
- digestive system: often – constipation; infrequently – vomiting, nausea; with an unknown frequency - pancreatitis;
- subcutaneous tissues and skin: with unknown frequency - Stevens-Johnson syndrome;
- urinary tract and kidneys: with unknown frequency - acute renal failure;
- biliary tract and liver: often - increased activity of liver enzymes; with an unknown frequency - hepatitis;
- general disorders: often – headache; infrequently – fatigue.
Adverse reactions, the development of which was noted during post-registration use of Maruxa: increased excitability and fatigue, drowsiness, dizziness, anxiety, headache, allergic reactions, increased intracranial and blood pressure, vomiting, nausea, muscle hypertonicity, hallucinations, impaired consciousness, convulsions, depression , gait disturbance, psychotic reactions, increased libido, suicidal thoughts, constipation, nausea, candidiasis, pancreatitis, cystitis, venous thrombosis, thromboembolism.
Overdose
Main symptoms: increased severity of adverse reactions - fatigue, weakness, diarrhea, confusion, drowsiness, dizziness, agitation, hallucinations, gait disturbance, nausea. In the most severe known case of overdose (2000 mg of memantine), the patient survived, but the development of nervous system disorders was noted (coma for 10 days, then agitation and diplopia). The patient received plasmapheresis and symptomatic therapy. No further complications were observed. In another reported case of severe overdose (400 mg), the patient also survived and recovered. Side effects from the central nervous system have been described: convulsive readiness, psychosis, anxiety, visual hallucinations, drowsiness, loss of consciousness and stupor.
Therapy: symptomatic. There is no specific antidote. Standard therapeutic measures are indicated that are aimed at removing the active substance from the stomach, in particular, taking activated charcoal, gastric lavage, acidifying urine, and forced diuresis may be prescribed.
special instructions
Since amantadine, ketamine or dextromethorphan (NMDA receptor antagonists) act on the same receptor system as Maruxa, adverse reactions (including nervous system disorders) when used together may occur more frequently and be more severe (combinations are recommended to be avoided).
In the presence of factors that influence the increase in urine pH, as well as renal tubular acidosis or severe urinary tract infections caused by Proteus spp., careful monitoring of the patient's condition is required. In addition, special attention is required when using Maruxa in patients with a history of myocardial infarction, decompensated chronic heart failure (III-IV functional class according to the NYHA classification) or uncontrolled arterial hypertension, which is associated with a lack of experience in use.
Impact on the ability to drive vehicles and complex mechanisms
With Alzheimer's disease at the stage of moderate to severe dementia, the ability to drive vehicles is usually impaired. Maruxa can also influence changes in reaction speed, and therefore patients should avoid driving.
Drug interactions
- antidepressants, selective serotonin reuptake inhibitors and monoamine oxidase inhibitors: during combined use, careful monitoring of the patient's condition is required;
- levodopa, dopamine receptor agonists and anticholinergic drugs: potentiation of effects;
- barbiturates, neuroleptics: their effectiveness decreases;
- amantadine, phenytoin, ketamine, dextromethorphan: the risk of developing psychosis increases; the combination is not recommended;
- dantrolene, baclofen, antispasmodics: their effect varies, which may require dose adjustment;
- cimetidine, ranitidine, procainamide, quinidine, quinine, nicotine: the likelihood of an increase in the plasma concentration of memantine in the blood increases;
- oral indirect anticoagulants (warfarin): increased MHO (international normalized ratio), control of prothrombin time and MHO is required;
- hydrochlorothiazide: its plasma concentration in the blood decreases (associated with an increase in its excretion from the body).
Interaction with other drugs
The effects of levodopa, dopamine receptor agonists and anticholinergic drugs are potentiated.
The effectiveness of barbiturates and antipsychotic (neuroleptic) drugs decreases with the simultaneous use of memantine.
The simultaneous use of memantine with dantrolene and baclofen, as well as with antispasmodics, may be accompanied by a change in their effect, which requires dose adjustment of these drugs.
The simultaneous use of memantine and amantadine should be avoided due to the risk of developing psychosis. Memantine and amantadine belong to the group of NMDA receptor antagonists. The risk of developing psychosis is also increased when memantine is used concomitantly with phenytoin, ketamine and dextromethorphan.
When used simultaneously with cimetidine, ranitidine, procainamide, quinidine, quinine and nicotine, the risk of increasing the concentration of memantine in the blood plasma increases.
When taken simultaneously with hydrochlorothiazide, it is possible to reduce the concentration of hydrochlorothiazide in the blood plasma due to an increase in its excretion from the body. It is possible to increase the International Normalized Ratio (INR) in patients simultaneously taking oral indirect anticoagulants (warfarin). It is recommended to regularly monitor prothrombin time or INR. Concomitant use with antidepressants, selective serotonin reuptake inhibitors and monoamine oxidase inhibitors requires careful monitoring of patients.
No drug interactions were observed with single simultaneous use of memantine with glibenclamide/metformin or donepizil in healthy volunteers.
When used simultaneously with memantine, no changes in the pharmacokinetics of galantamine were observed in healthy volunteers.
Under in vitro conditions, memantine does not inhibit CYP isoenzymes 1A2, 2A6, 2C9, 2D6, 2E1, 3A, flavin-containing monooxygenase, epoxide hydrolase, or sulfation.