Instructions for use METOCLOPRAMIDUM
In patients with a history of depression, especially moderate or severe depression, accompanied by suicidal tendencies, a relapse of the disease may occur during treatment with metoclopramide. Before starting treatment, it is necessary to weigh the ratio of the potential benefits of treatment to the possible risks.
During the first 24-48 hours of treatment with metoclopramide, extrapyramidal symptoms may appear in the form of disturbances in muscle tone, dystonic movements, paroxysmal dystonia, and spasmodic torticollis. These symptoms most often occur in children and people under 30 years of age, and also after using metoclopramide to prevent vomiting caused by cancer chemotherapy.
During the first 6 months of treatment with metoclopramide, symptoms of parkinsonism may appear. These symptoms disappear within 2-3 months after discontinuation of the drug product.
Elderly patients, especially women, treated with metoclopramide may experience dyskinesias, often irreversible. These symptoms occur less frequently during short-term treatment using lower doses of the drug product.
In patients with impaired renal function, hypokalemia may occur during treatment with metoclopramide because the drug increases plasma aldosterone concentrations and decreases sodium excretion.
In patients with hypertension treated with MAO inhibitors, metoclopramide potentiates the effect of MAO inhibitors.
Metoclopramide causes a transient increase in plasma aldosterone levels. This can lead to fluid retention in the body, especially in patients with cirrhosis or congestive heart failure. If these symptoms are observed during the first weeks of treatment with metoclopramide, the drug should be discontinued.
In vitro studies have shown that about 1/3 of breast tumors depend on prolactin levels. Metoclopramide increases prolactin levels, so the drug should be used with great caution in patients with a history of breast neoplasm.
IV administration of diluted metoclopramide should continue for at least 15 minutes.
Influence on the ability to control transport or other mechanisms
Metoclopramide may cause CNS side effects such as:
- drowsiness and dizziness, which impair psychophysical performance. Therefore, it is necessary to inform the patient about the dangers associated with driving motor vehicles and servicing moving mechanisms.
Metoclopramide
Directions for use and doses
Intravenously or intramuscularly.
The intravenous drug should be administered as a slow bolus (at least 3 minutes). To avoid overdose, it is necessary to observe a minimum interval of 6 hours between administrations of the drug.
Adults
Prevention of postoperative nausea and vomiting
The recommended single dose is 10 mg.
Symptomatic treatment of nausea and vomiting, including acute migraine. Prevention of nausea and vomiting caused by radiation therapy and chemotherapy.
The recommended single dose of 10 mg is administered up to three times a day.
To enhance peristalsis during X-ray contrast studies of the gastrointestinal tract
A slow intravenous bolus of 10-20 mg is recommended 10 minutes before the start of the study.
The maximum daily dose is 30 mg or 0.5 mg/kg.
The period of administration of the drug in the form of an injection should be as short as possible, followed by a transition to the dosage form for oral administration.
Children aged from 1 year to 18 years. Slow intravenous bolus administration is recommended. The dose is calculated in accordance with the table or based on the calculation of 0.10-0.15 mg/kg body weight up to 3 times a day. The maximum daily dose is 0.5 mg/kg body weight.
| Age (in years) | Weight, kg) | Dose (mg) | Frequency |
| 1-3 | 10-14 | 1 | Up to 3 times a day |
| 3-5 | 15-19 | 2 | Up to 3 times a day |
| 5-9 | 20-29 | 2,5 | Up to 3 times a day |
| 9-18 | 30-60 | 5 | Up to 3 times a day |
| 15-18 | More than 60 | 10 | Up to 3 times a day |
The maximum duration of use of metoclopramide for the prevention of postoperative nausea and vomiting is 2 days.
To prevent nausea and vomiting caused by chemotherapy - 5 days.
In elderly patients, dose reduction may be required due to decreased renal and liver function.
In patients with end-stage renal failure (creatinine clearance less than 15 ml/min), the daily dose should be reduced by 75%. In patients with moderate or severe renal failure (creatinine clearance 15-60 ml/min), the dose should be reduced by 50%.
In patients with severe liver failure, the dose should be reduced by 50%.
Side effect
The frequency of adverse reactions is classified as follows: very often (? 1/10), often (? 1/100 - < 1/10), infrequently (? 1/1000 - < 1/100), rarely (? 1/10000 - < 1/1000), very rare (< 1 in 10000), frequency unknown (cannot be estimated from available data).
From the digestive system: at the beginning of treatment, constipation, diarrhea, nausea are possible; rarely - dry mouth.
From the central nervous system: at the beginning of treatment, a feeling of fatigue, drowsiness, dizziness, headache, depression, asthenia, extrapyramidal disorders (especially in children and young patients and/or when the recommended doses of the drug are exceeded, even after a single administration) are possible, infrequently - dystonia, disturbances of consciousness, rarely - seizures (especially in patients with epilepsy), frequency unknown - tardive dyskinesia, which may be permanent, during or after long-term treatment (especially in elderly patients), neuroleptic malignant syndrome. With long-term use, more often in elderly patients, parkinsonism and dyskinesia are possible.
Mental disorders: often - depression; infrequently - hallucinations; rarely - confusion.
From the hematopoietic and lymphatic systems: agranulocytosis is possible at the beginning of treatment; frequency unknown - methemoglobinemia, which may be associated with deficiency of the enzyme NADH-cytochrome b5 reductase, especially in newborns; sulfhemoglobinemia caused by sulfur-containing substances in the composition of the drug (mainly with the concomitant use of high doses of drugs containing sulfur); leukopenia, neutropenia.
From the heart: rarely - bradycardia; frequency unknown - cardiac arrest, which may be caused by bradycardia; atrioventricular block, sinus node block (especially with intravenous administration), prolongation of the QT interval on the electrocardiogram, torsade de pointes.
From the side of blood vessels: often - hypotension, especially with intravenous administration; frequency unknown - cardiogenic shock, syncope after injection, acute arterial hypertension in patients with pheochromocytoma.
From the endocrine system*: infrequently - amenorrhea, hyperprolactinemia; rarely - galactorrhea; frequency unknown - gynecomastia.
* Endocrine disorders during long-term treatment are associated with hyperprolactinemia (amenorrhea, galactorrhea, gynecomastia).
From the immune system: infrequently - hypersensitivity; frequency unknown - anaphylactic reactions (including anaphylactic shock); Allergic reactions: rarely - urticaria, maculopapular rash. From the kidneys and urinary tract : frequency unknown - polyuria, urinary incontinence. From the genital organs and breast: unknown frequency - sexual dysfunction, priapism.
Adverse reactions that are most common when using high doses of the drug metoclopramide: extrapyramidal symptoms: acute dystonia and dyskinesia, parkinsonism syndrome, akathisia developed even after using a single dose of the drug, especially in children and young patients (see section “Special instructions”); drowsiness, decreased level of consciousness, confusion, hallucinations.
Overdose
Symptoms. Disorientation and extrapyramidal disorders, hypersomnia, drowsiness, changes in consciousness, confusion and hallucinations, irritability, dizziness, bradycardia, changes in blood pressure, cardiac and respiratory arrest, abdominal pain. As a rule, symptoms disappear after stopping the drug for 24 hours.
Treatment . In the event of the development of extrapyramidal symptoms caused by overdose or for another reason, treatment is exclusively symptomatic (benzodiazepines in children and/or anticholinergic antiparkinsonian drugs in adults).
Symptomatic treatment and constant monitoring of cardiovascular and respiratory function are required depending on the clinical condition of the patient. There is no specific antidote.
Interactions with other drugs
Contraindicated combinations. The simultaneous use of metoclopramide with levodopa or dopamine receptor agonists is contraindicated due to the existing mutual antagonism.
Combinations to avoid . Alcohol enhances the sedative effect of metoclopramide.
Combinations requiring caution . Due to the prokinetic effect of metoclopramide, the absorption of some drugs may be impaired.
M-anticholinergic agents and morphine derivatives have mutual antagonism with metoclopramide in terms of their effect on gastrointestinal motility.
Medicines that depress the central nervous system (morphine derivatives, tranquilizers, H1-histamine blockers, antidepressants with sedative effects, barbiturates, clonidine and other drugs of these groups) can enhance the sedative effect under the influence of metoclopramide.
Metoclopramide enhances the effect of antipsychotics on extrapyramidal symptoms. With the concomitant use of metoclopramide and tetrabenazine orally, there is a possibility of dopamine deficiency, which may be accompanied by muscle spasms, difficulties in speaking or swallowing, anxiety, tremor, involuntary muscle movements, including facial muscles.
The use of metoclopramide with serotonergic drugs, such as selective serotonin reuptake inhibitors, increases the risk of developing serotonin syndrome (serotonin toxicity).
Metoclopramide reduces the bioavailability of digoxin, and monitoring of digoxin plasma concentrations is required.
Metoclopramide enhances the absorption of tetracycline, ampicillin, paracetamol, acetylsalicylic acid, mexiletine, lithium, levodopa, ethanol and cyclosporine (Cmax by 46% and exposure by 22%, which requires monitoring of the concentration of cyclosporine in the blood plasma); reduces the absorption of cimetidine.
Metoclopramide exposure increases when used concomitantly with strong CYP2D6 inhibitors, such as fluoxetine and paroxetine. Although the clinical significance of this interaction has not been established, patients should be monitored for adverse reactions.
With the concomitant use of metoclopramide with atovaquone, the concentration of atovaquone in the blood plasma is significantly reduced (about 50%). Concomitant use of metoclopramide with atovaquone is not recommended.
With the concomitant use of metoclopramide with bromocriptine, the concentration of bromocriptine in the blood plasma increases.
Metoclopramide solution is pharmaceutically (physically and chemically) compatible (up to 48 hours) with solutions of cimetidine, mannitol, potassium acetate and potassium phosphate; physically compatible (up to 48 hours) with solutions of ascorbic acid, benztropine mesylate, cytarabine, dexamethasone sodium phosphate, diphenhydramine, doxorubicin, sodium heparin, hydrocortisone sodium phosphate, lidocaine hydrochloride, solutions of multivitamins (if stored in the refrigerator), solutions of B vitamins, with ascorbic acid.
Metoclopramide solution is physically compatible for up to 24 hours (do not use if precipitation occurs) with clindamycin phosphate, cyclophosphamide, insulin. Conditionally compatible (use within one hour of mixing or can be infused directly into the same venous line) with ampicillin sodium, cisplatin, erythromycin lactobionate, methotrexate sodium, benzylpenicillin potassium, tetracycline hydrochloride. Incompatible (do not combine!) with cephalothin sodium, chloramphenicol sodium, sodium bicarbonate.
special instructions
Metoclopramide is not effective against vomiting of vestibular origin.
Due to the sodium sulfite content, metoclopramide injection solution should not be prescribed to patients with bronchial asthma or hypersensitivity to sulfites.
During the use of metoclopramide, distortion of data on laboratory parameters of liver function and determination of the concentration of aldosterone and prolactin in plasma is possible.
Most side effects occur within 36 hours of starting treatment and disappear within 24 hours after discontinuation. Treatment should be short-term if possible.
During treatment with the drug, alcohol consumption is not recommended.
Impact on the ability to drive vehicles and machinery
During the treatment period, it is necessary to refrain from driving vehicles, operating machinery and other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Metoclopramide-Vial, solution 5 mg/ml, ampoules 2 ml, 10 pcs.
When used simultaneously with anticholinergic drugs, mutual weakening of the effects is possible.
When used simultaneously with antipsychotics (especially phenothiazines and butyrophenone derivatives), the risk of extrapyramidal reactions increases.
With simultaneous use, the absorption of acetylsalicylic acid, paracetamol, and ethanol is enhanced.
Metoclopramide, when administered intravenously, increases the rate of absorption of diazepam and increases its maximum concentration in blood plasma.
When used simultaneously with a slowly dissolving dosage form of digoxin, it is possible to reduce the concentration of digoxin in the blood serum by 1/3. When used concomitantly with digoxin in liquid or instant dosage form, no interaction was observed.
When used simultaneously with zopiclone, the absorption of zopiclone is accelerated; with cabergoline - the effectiveness of cabergoline may be reduced; with ketoprofen - the bioavailability of ketoprofen decreases.
Due to antagonism of dopamine receptors, metoclopramide may reduce the antiparkinsonian effect of levodopa, while the bioavailability of levodopa may be increased due to the acceleration of its evacuation from the stomach under the influence of metoclopramide. The results of the interaction are mixed.
When used simultaneously with mexiletine, the absorption of mexiletine is accelerated; with mefloquine - the rate of absorption of mefloquine and its concentration in the blood plasma increases, while its side effects may be reduced.
When used simultaneously with morphine, the absorption of morphine when taken orally is accelerated and its sedative effect is enhanced.
When used simultaneously with nitrofurantoin, the absorption of nitrofurantoin is reduced.
When using metoclopramide immediately before the administration of propofol or thiopental, it may be necessary to reduce their induction doses.
In patients receiving metoclopramide, the effects of suxamethonium chloride are enhanced and prolonged.
When used simultaneously with tolterodine, the effectiveness of metoclopramide decreases; with fluvoxamine - a case of the development of extrapyramidal disorders is described; with fluoxetine - there is a risk of developing extrapyramidal disorders; with cyclosporine - the absorption of cyclosporine increases and its concentration in the blood plasma increases.
Directions for use and doses
The injection solution is administered intravenously or intramuscularly to adults at a dose of 10-20 mg (maximum daily dose - 60 mg); children over 6 years old - 5 mg 1-3 times a day; for children under 6 years of age, the daily dose is 0.5-1 mg/kg, the frequency of administration is 1-3 times.
For the prevention and treatment of nausea and vomiting caused by the use of cytostatics or radiation therapy, the drug is administered intravenously at a dose of 2 mg/kg 30 minutes before the use of cytostatics or radiation; if necessary, the administration is repeated after 2-3 hours.
Metoclopramide solution for intravenous and intramuscular administration 5 mg/ml in 2 ml ampoules No. 10
Name
Metoclopramide solution d/in. 5 mg/ml in amp. 2 ml in pack No. 10
Description
For adults. For the prevention of postoperative nausea and vomiting. For the symptomatic treatment of nausea and vomiting, including nausea and vomiting due to acute migraine. For the prevention of nausea and vomiting induced by radiation therapy. The injection course of treatment should be as short as possible. The patient should be transferred to the oral or rectal route of administration as soon as possible. Children aged 1 to 18 years. For the prevention of delayed (non-acute) nausea and vomiting caused by chemotherapy, as a second-line drug. The maximum course of treatment is 5 days. For the treatment of established postoperative nausea and vomiting, as a second-line drug. The maximum course of treatment is 48 hours.
Main active ingredient
metoclopramide hydrochloride
Release form
Solution
Dosage
5mg/ml
Directions for use and doses
The maximum duration of use of the drug is no more than 5 days! The injection solution is administered intramuscularly or intravenously as a bolus over at least 3 minutes. Adults at a dose of 10 mg up to 3 times a day (maximum single dose – 10 mg, maximum daily dose – 30 mg or 0.5 mg/kg). For children. The dose is calculated in accordance with the table or based on the calculation of 0.10 - 0.15 mg/kg body weight up to 3 times a day. The maximum daily dose is 0.5 mg/kg body weight. The maximum duration of therapy is 5 days. In case of repeated vomiting, the minimum interval between administrations of metoclopramide should not be less than 6 hours. In case of reduced renal function, the drug is prescribed: - with creatinine clearance less than 15 ml/min - in doses reduced by 75%; - with creatinine clearance from 15 to 60 ml/min - in doses reduced by 50%. In severe liver failure, the dose of metoclopramide should be reduced by 50%. In elderly patients, dosing is carried out taking into account changes in liver and kidney function, as indicated above.
Use during pregnancy and lactation
Forbidden.
Contraindications
Hypersensitivity to metoclopramide or components of the drug, gastrointestinal bleeding, pyloric stenosis, mechanical intestinal obstruction, perforation of the stomach or intestines, 3 - 4 days after surgery on the stomach and / or intestines, pheochromocytoma (confirmed or suspected, due to the risk of severe hypertensive complications), Parkinson's disease, extrapyramidal disorders (including a history of neuroleptic or metoclopramide-induced tardive dyskinesia), epilepsy, prolactin-dependent tumors, a history of episodes of methemoglobinemia while taking metoclopramide or with NADP-cytochrome-b5 deficiency, concomitant use of levodopa or dopamine stimulants receptors, pregnancy, children under 1 year of age, lactation period. Due to the sodium sulfite content, metoclopramide solution should not be prescribed to patients with bronchial asthma with hypersensitivity to sulfite.
Compound
one ampoule (2 ml of solution) contains - active ingredient: metoclopramide hydrochloride - 10 mg; excipients: sodium chloride, sodium sulfite anhydrous E 221, disodium edetate, propylene glycol, hydrochloric acid, water for injection.
Buy Metoclopramide solution for IV and IM administration. 5 mg/ml in amp. 2 ml in pack No. 10 in the pharmacy
Price for Metoclopramide solution for intravenous and intramuscular injection. 5 mg/ml in amp. 2 ml in pack No. 10
Instructions for use for Metoclopramide solution for intravenous and intramuscular administration. 5 mg/ml in amp. 2 ml in pack No. 10
pharmachologic effect
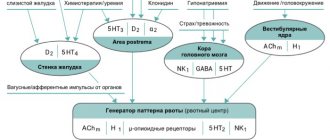
An antiemetic, it is a specific blocker of dopamine (D2) and serotonin receptors. Eliminates nausea and hiccups. Reduces motor activity of the esophagus, increases the tone of the lower esophageal sphincter, accelerates gastric emptying, and also accelerates the movement of food through the small intestine without causing diarrhea. Stimulates the secretion of prolactin. The onset of action on the gastrointestinal tract is observed after 1-3 minutes. after intravenous administration and 10-15 minutes after intramuscular administration and is manifested by accelerated evacuation of gastric contents and an antiemetic effect.
Indications for use
- Vomiting, nausea, hiccups of various origins (in some cases it can be effective for nausea and vomiting caused by the use of cytostatics).
- atony and hypotension of the stomach and intestines (in particular, postoperative);
- biliary dyskinesia;
- reflux esophagitis; flatulence, exacerbation of gastric and duodenal ulcers (as part of complex therapy).
- used to enhance peristalsis during X-ray contrast studies of the gastrointestinal tract, and also as a means of facilitating duodenal intubation.