pharmachologic effect
Manufacturer: MADAUS GMBH (Germany)
Release form: tablets, capsules, injection solution, powder in bags
Active ingredient: glucosamine
Analogs: Mukosat, Alflutop, Arthra
Dona belongs to the group of stimulators of bone and cartilage tissue repair, which has anti-inflammatory and anesthetic effects. The product stimulates the permeability of the joint capsule, increases the efficiency of enzymatic processes in the structures of cartilage and synovial membrane.
Under the influence of the drug, the process of normalizing calcium deposition in bone tissue, slowing down the progression of degenerative changes in joints, reducing pain and increasing the amplitude of active movements occurs.
Pharmacological properties
Pharmacodynamics.
The active ingredient, glucosamine sulfate, is a salt of the aminomonosaccharide glucosamine, which is present in the human body under physiological conditions and is used together with sulfates for the biosynthesis of hyaluronic acid, synovial fluid and glycosaminoglycans of the main substance of articular cartilage. Thus, the mechanism of action of glucosamine sulfate is to stimulate the synthesis of glycosaminoglycans and, accordingly, articular proteoglycans. In addition, glucosamine has an anti-inflammatory effect and suppresses the process of destruction of articular cartilage mainly due to the possible manifestations of its own metabolic properties, the ability to suppress the activity of interleukin 1 (IL-1), which, on the one hand, affects the symptoms of osteoarthritis, and on the other - potentially delays structural damage to joints, as evidenced by data from long-term clinical studies.
According to initial studies in vitro and in vivo, exogenous administration of glucosamine sulfate stimulates the biosynthesis of proteoglycans, which is insufficient in osteoarthritis, promotes the fixation of sulfur ions during the synthesis of glycosaminoglycans and improves the trophism of articular cartilage.
Subsequent studies have shown that glucosamine sulfate inhibits the synthesis of substances that destroy tissue, such as superoxide radicals, as well as the activity of lysosomal enzymes, in addition to enzymes that can destroy articular cartilage tissue, such as collagenases and phospholipases A2. This action produces a modest anti-inflammatory effect that is observed in in vivo animal models, including some cases of experimental osteoarthritis, even without COX inhibition, unlike NSAIDs.
More recent studies have shown that most of the above metabolic and anti-inflammatory effects may be associated with inhibition of intracellular signal transduction stimulating IL-1, one of the cytokines involved in the pathogenesis of osteoarthritis, with subsequent inhibition of cytokine-induced gene transcription. Glucosamine sulfate, at concentrations in plasma and synovial fluid described in patients with osteoarthritis, may actually inhibit IL-1-induced gene expression of a series of proinflammatory enzymes in joint tissue, as well as prodegenerative enzymes in cartilage, such as certain metalloproteases, including aggrecanases. The possible potential influence of sulfur ions on the mentioned pharmacodynamic properties of glucosamine has not been fully elucidated.
All of the above properties have a beneficial effect on degenerative processes in cartilage, which underlie the pathogenesis of osteoarthritis, as well as on the clinical picture of the disease.
Short-term and intermediate-term studies have shown that glucosamine sulfate is effective against osteoarthritis symptoms within 2 to 3 weeks of use.
On the other hand, the effectiveness of treatment with glucosamine sulfate in relation to symptoms compared with conventional analgesics and NSAIDs is optimal after a course of continuous use for 6 months or after a course of use for 3 months with an obvious aftereffect within 2 months after discontinuation.
Results from clinical studies of daily continuous treatment over 3 years show a progressive increase in its effectiveness in terms of symptoms and slowing of structural damage to the joints, as confirmed by X-ray examination.
Glucosamine sulfate demonstrated good tolerability. No significant effects of glucosamine sulfate on the cardiovascular, respiratory, autonomic or central nervous system have been identified.
Pharmacokinetics. Studies in humans and animals have shown that following oral administration of 14C-glucosamine, the radiolabeled elements are rapidly and almost completely absorbed systemically. In humans, about 90% of the radioactively labeled dose of the drug is absorbed. The bioavailability of glucosamine in rats after oral administration of glucosamine sulfate was 26% due to the first pass effect through the liver. The absolute bioavailability in humans is unknown, but according to allometric calculations it is similar to that observed in rats, i.e. 20 to 30%.
In healthy volunteers, after repeated oral administration of glucosamine sulfate at a dose of 1500 mg/day, the equilibrium Cmax, ss in blood plasma was 1602 ± 425 ng/ml (8.9 μM). This concentration was achieved 1.5–4 hours (median 3 hours) after administration (tmax). At steady state, the AUC of plasma concentrations versus time was 14,564±4138 ng·h/ml. These parameters were obtained when using the drug on an empty stomach, so it is unknown whether food intake can significantly affect the absorption of the drug.
When administered orally, once absorbed, glucosamine is primarily distributed into the extravascular environment (including synovial fluid), with a volume of distribution approximately 37 times greater than total body water. Glucosamine binding to proteins has not been detected.
The metabolic profile of glucosamine was not studied because this drug, being a natural substance present in the human body, is used for the biosynthesis of some components of articular cartilage.
Only the terminal elimination T½ of glucosamine from human plasma has been established based on the results of a study of glucosamine levels in blood plasma, which were measured within 48 hours after dosing. The calculated value was about 15 hours.
After oral administration of 14C-glucosamine, urinary excretion of radiolabeled elements in humans was 10±9% of the administered dose, while fecal excretion was 11.3±0.1%. Urinary excretion of unchanged glucosamine in humans after oral administration was on average low (about 1% of the administered dose). These results indicate that the kidneys do not play a significant role in the excretion of glucosamine and/or its metabolites and/or its breakdown products.
When administered at repeated doses of 750–1500 mg/day, the pharmacokinetics of glucosamine were linear, whereas when administered at a dose of 3000 mg, plasma glucosamine levels were lower than expected based on dose escalation. The pharmacokinetics of glucosamine at steady state were independent of time, without accumulation or decrease in bioavailability, compared to the pharmacokinetic profile observed after a single dose.
The pharmacokinetics of glucosamine in men and women are similar; no differences in pharmacokinetics have been established in healthy volunteers and in patients with osteoarthritis of the knee joint. In the latter, the average concentration in blood plasma 3 hours after taking the last dose of 1500 mg with repeated use 1 time per day was 7.2 μM and was similar to that found in healthy volunteers, while the average concentration in synovial fluid was only 25% lower and therefore also in the 10 μM range. In patients with renal or hepatic insufficiency, the pharmacokinetics of glucosamine have not been studied, since, given the safety profile of the drug, and due to the insignificant participation of the kidneys in the elimination of glucosamine, dose reduction in these groups of patients is not provided.
Steady-state concentrations of glucosamine in plasma and synovial fluid after repeated once-daily administration of 1500 mg are in the range of 10 μM and, therefore, correspond to those for which pharmacological activity has been shown in studies in experimental in vitro models, which confirms the mechanism of action and clinical effect of the drug.
Dona - instructions for use
According to the instructions for use of Dona, the medicine is prescribed 1 tablet 2 times a day, mainly during meals, which is washed down with a sufficient volume of water. The duration of the course of therapy can be up to 1.5 months. It is possible to adjust the dosage of the drug upward according to indications.
For intramuscular administration, the product solution is mixed with a solvent in 1 syringe and 3.0 ml is administered 3 times a week for 30–45 days. A packet of powder is dissolved in 200 ml of water and taken once a day for up to 3 months.
Dona solution for intramuscular administration 200 mg/ml in 2 ml ampoules complete with solvent No. 6
Name
Don.
Description
Solution A is a clear, colorless liquid or a clear, light brown liquid that does not contain suspended particles; solution B is a clear, colorless liquid that does not contain suspended particles; solution A plus solution B is a light brown transparent solution that does not contain suspended particles.
Main active ingredient
Release form
Solution for intramuscular administration.
Dosage
200 mg / 1 ml 2 ml.
special instructions
pharmachologic effect
Other nonsteroidal anti-inflammatory and antirheumatic drugs. ATX code: M01AX05.
Pharmacodynamics
Mechanism of action. Glucosamine sulfate is a salt of the amino monosaccharide glucosamine, which is an endogenous component and preferred substrate for the synthesis of glycosaminoglycans and proteoglycans in articular cartilage and synovial fluid. Glucosamine sulfate inhibits the activity of interleukin-1 beta and other inflammatory mediators. Clinical efficacy and tolerability The safety and effectiveness of glucosamine sulfate has been confirmed in clinical trials with treatment durations (oral administration) of up to three years. Short- and medium-term clinical studies have shown that the effectiveness of glucosamine sulfate in relation to the symptoms of osteoarthritis is observed after 2-3 weeks of its use. However, unlike NSAIDs, glucosamine sulfate has a long-lasting effect, lasting from 6 months to 3 years. Clinical studies with daily administration of glucosamine sulfate for up to 3 years have shown gradual improvement in symptoms and slowing of structural changes in the joint, as demonstrated by radiography. Glucosamine sulfate demonstrated good tolerability during short-term and long-term courses of treatment. Evidence of the drug's effectiveness was demonstrated when taken for three months, with residual effects for two months after its discontinuation. The safety and effectiveness of the drug have also been confirmed in clinical trials for up to three years. Continuous treatment for more than 3 years cannot be recommended, since there are no safety data when taking glucosamine for more than 3 years.
Pharmacokinetics
Glucosamine is a relatively small molecule (molecular weight 179) that is readily soluble in water and hydrophilic organic solvents. Available information on the pharmacokinetics of intramuscularly administered glucosamine is limited. Absolute bioavailability is unknown. The volume of distribution is approximately 5 liters and the half-life after intravenous administration is approximately 2 hours. Approximately 38% of an intravenously administered dose is excreted unchanged in the urine.
Indications for use
Relief of symptoms (mild to moderate pain) of adequately diagnosed osteoarthritis of the knee.
Directions for use and doses
For intramuscular use! The drug is not intended for intravenous administration. Adult and elderly patients Before use, mix solution B (solvent 1 ml) with solution A (drug solution 2 ml) in one syringe. The prepared solution of the drug is administered intramuscularly in 3 ml or 6 ml (solution A - B) 3 times a week for 4-6 weeks. The presence of a yellowish color of the solution in ampoule A does not affect the effectiveness and tolerability of the drug. Injections of the drug can be combined with oral administration of the drug in powder form to prepare a solution. Glucosamine is not intended for the treatment of acute pain. Symptom relief (especially pain relief) may occur after only a few weeks of treatment, and in some cases even after a longer time. If no relief of symptoms occurs after 2–3 months of use, treatment should be reconsidered. Patients should consult a doctor if pain worsens after starting glucosamine. Dosage regimen for different categories of patents Elderly patients No dose adjustment is required. Patients with impaired renal and/or liver function In patients with impaired renal and/or liver function, no recommendations for dose adjustment exist, since relevant studies have not been conducted. Children and adolescents Glucosamine should not be used in children and adolescents under the age of 18 years, since there is no data on the safety and effectiveness of glucosamine in this category of patients.
Use during pregnancy and lactation
There are no data regarding the use of the drug in pregnant women or during breastfeeding, so use of the drug during pregnancy or breastfeeding is not recommended.
Impact on the ability to drive vehicles and other mechanisms
Studies regarding the effect of the drug on the ability to drive a car and other mechanisms have not been conducted. You should be careful when driving vehicles and performing work that requires attention. If you experience headache, drowsiness, fatigue, dizziness or visual impairment, driving or operating other machinery is not recommended.
Precautionary measures
It is necessary to consult a doctor to exclude the presence of joint disease, for which other treatment methods are provided. Cases of exacerbation of bronchial asthma symptoms have been described after starting to take glucosamine. Patients suffering from bronchial asthma should be informed about the possible worsening of symptoms of the disease. One dose of the drug contains 40.3 mg of sodium. This should be taken into account when prescribing to patients on a sodium-restricted diet. Patients with impaired glucose tolerance should use caution when taking glucosamine. For patients with diabetes mellitus, it is recommended that blood sugar levels be monitored and insulin requirements determined before and periodically during treatment. No specific studies have been conducted in patients with renal or hepatic impairment. The toxicological and pharmacokinetic profile of glucosamine does not suggest restrictions for these patients. However, the use of glucosamine in patients with severe hepatic or renal impairment should be carried out under medical supervision.
Interaction with other drugs
No specific drug interaction studies have been conducted. There is evidence of an enhanced effect of coumarin anticoagulants; therefore, more careful monitoring of coagulation parameters is necessary in patients simultaneously taking coumarin anticoagulants (for example, warfarin or acenocoumarol). Oral administration of glucosamine sulfate may increase the absorption of tetracyclines from the gastrointestinal tract, but the clinical significance of this interaction is small. It is acceptable to take steroidal or non-steroidal anti-inflammatory drugs simultaneously with glucosamine.
Contraindications
Individual hypersensitivity to the active substance or to any of the excipients. The drug should not be used by patients with allergies to shellfish, since the active substance (glucosamine) is obtained from the shells of mollusks and crustaceans. Pregnancy and breastfeeding period. The injection form of the drug contains the excipient lidocaine, which has the following contraindications: arrhythmia, acute heart failure, and hypersensitivity to lidocaine.
Compound
Solution A: each ampoule (2 ml) contains: active substance: crystalline glucosamine sulfate 502.5 mg (contains 400 mg glucosamine sulfate and 102.5 mg sodium chloride); excipients: lidocaine hydrochloride 10.0 mg, water for injection up to 2 ml. Solution B: ampoule volume (1 ml) contains: excipients: diethanolamine – 24.0 mg, water for injection up to 1 ml.
Overdose
Cases of overdose are unknown. In cases of overdose, symptomatic treatment should be carried out aimed at restoring water and electrolyte balance.
Side effect
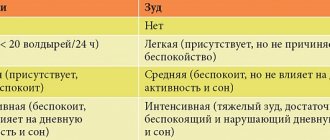
To determine the frequency of adverse events, the following categories of their occurrence in patients were used: Very often (≥ 1/10) Often (≥ 1/100 to
Storage conditions
At a temperature not exceeding 25 °C. Keep out of the reach of children.
Buy Dona solution for intramuscular injection. 200 mg/ml in amp 2 ml in com. with solution (diethane, water for injection in amp 1 ml) in a pack. No. 6 in the pharmacy
Price for Dona solution for intramuscular injection. 200 mg/ml in amp 2 ml in com. with solution (diethane, water for injection in amp 1 ml) in a pack. No. 6
Instructions for use for Don solution for intramuscular injection. 200 mg/ml in amp 2 ml in com. with solution (diethane, water for injection in amp 1 ml) in a pack. No. 6
Analogues of Dona
The pharmaceutical market is represented by a large number of analogues of the drug Don, which have a similar therapeutic effect, but may differ in some parameters. You can purchase Dona substitutes at the pharmacy in the form of:
- synonyms;
- generics;
- combined means.
The price category of these medications has a wide range. There are analogs of Dona that are cheaper and more expensive, but with an equivalent therapeutic result.
Table of Dona analogues with price and country of origin
| Analogue | Cost in rubles | Manufacturer country |
| Don | 1150-2380 | Germany |
| Mucosat | 250-1500 | Russia |
| Alflutop | 1700-2300 | Romania |
| Teraflex | 1700-3600 | Germany |
| Artra | 750-1700 | USA |
| Sustilak | 1000-1200 | India |
| Structum | 1000-1400 | France |
| Rumalon | 1500-2600 | Bulgaria |
| Elbona | 1055-1260 | Russia |
| Chondrogard | 650-1450 | Russia |
| Chondrolone | 700-950 | Russia |
A higher price for Dona analogues is observed among foreign manufacturers.
What else can replace Don, what effective analogues for the treatment of osteoarticular pathology? The list can be supplemented with the following medications:
- Glucosamine;
- Drastop;
- Sustagard arthro;
- Piaskledin;
- Artrocam;
- Injectran;
- Diaflex;
- Chondroitin;
- Flexamine;
- Sinagra.
A wide range of Dona analogs allows for effective treatment of diseases of the osteoarticular system not only for adults, but also for children.
Dona or Mukosat - which is better?
Manufacturer: JSC SINTEZ (Russia)
Release form: capsules, solution for intramuscular and intraarticular administration
Active ingredient: chondroitin sulfate
Mucosat is an analogue of Dona tablets. Both products are chondroprotectors that improve the structure of cartilage tissue and slow down degenerative processes in it. But their mechanism of action is different due to the difference in active substances.
The best drug would be Mucosat, since the analogue is well tolerated, has minimal and rare side effects and broader indications. It can be used in fractures to stimulate callus formation. Patients often choose Mucosat, since this analogue is cheaper than Don tablets, which is an important point during a long course of treatment.
special instructions
Don injection solution can only be administered by medical professionals.
In patients with asthma, the drug should be used with caution, since such patients may be more prone to developing allergic reactions to glucosamine with a possible exacerbation of the symptoms of their disease.
Powder for oral use contains sorbitol. In patients with a rare hereditary form of fructose intolerance, the drug in this release form is not recommended.
1 sachet contains 6.6 mmol (151 mg) sodium, 1 dose of the drug in the form of an injection solution contains 40.3 mg sodium. This should be taken into account in patients on a sodium-controlled diet.
At the beginning of treatment for patients with diabetes, it is advisable to monitor blood glucose levels. Dona solution for injection should be prescribed with caution to patients with glucose intolerance.
Dona powder for oral use should only be used under medical supervision in patients with impaired liver and kidney function, thrombophlebitis.
Considering that the injection form of the drug contains the excipient lidocaine, before using it it is necessary to conduct a skin test for individual sensitivity to the drug, which is indicated by swelling and redness of the injection site. To avoid accidental intravasal administration of the drug, it is recommended to perform an aspiration test.
The safety of using anesthetics of the lidocaine group is questionable in patients prone to malignant hyperthermia, so their use in such cases should be avoided.
Before using lidocaine for heart disease (hypokalemia reduces the effectiveness of lidocaine), it is necessary to normalize the level of potassium in the blood and monitor the ECG.
CPK activity in the blood serum may increase after intramuscular injection of the drug, which may lead to an error in establishing the diagnosis of acute myocardial infarction.
In case of dysfunction of the sinus node, prolongation of the P-Q interval, expansion of the QRS complex, or in the event of the occurrence or exacerbation of arrhythmia, the dose should be reduced or the drug should be discontinued.
Particular caution should be exercised when using the drug in patients with circulatory failure, arterial hypotension, a history of arrhythmia, and moderate liver and/or kidney dysfunction. Due to the presence of lidocaine in the composition, caution must also be exercised when prescribing to elderly patients, patients with epilepsy, cardiac conduction disorders, and respiratory failure.
Use during pregnancy and lactation. There are no data on the use of the drug during pregnancy and breastfeeding, therefore the use of the drug in this category of patients is contraindicated.
The ability to influence reaction speed when driving vehicles or other mechanisms. Studies of the effect of the drug on the ability to drive vehicles and other mechanisms have not been conducted. Caution should be exercised when operating vehicles or performing work that requires attention. In case of drowsiness, fatigue, dizziness or visual impairment, driving vehicles and operating machinery is prohibited.
Dona or Alflutop - which is better and more effective
Manufacturer: K.O.
BIOTECHNOS S.A. (Romania) Release form: solution for injection and intra-articular administration
Active ingredient: bioactive concentrate from small marine fish
Dona and Alflutop have chondroprotective, anti-inflammatory and analgesic effects, and have a positive effect on local metabolism in cartilage and bone tissue. This analogue of Dona in injections will be better and more effective for acute pain syndrome accompanying diseases of the joints or spine.
The drug has a rapid effect, especially when injected into a joint, which leads to a decrease in pain and an increase in the range of motion of the limb. This effect of using the analogue is confirmed by reviews from doctors.
Side effects
Criteria for assessing the frequency of adverse drug reactions: very often - ≥1/10, often - from ≥1/100 to ≤1/10, infrequently - from ≥1/1000 to ≤1/100, rarely - from ≥1/10 000 to ≤1/1000, very rare - ≤1/10,000, unknown - frequency cannot be estimated from available data.
From the digestive system: often - abdominal pain, flatulence, dyspepsia, diarrhea, constipation, nausea, vomiting; unknown - hyperglycemia in patients with impaired glucose tolerance;
from the nervous system: often - headache, drowsiness, increased fatigue, dizziness;
from the immune system: unknown - hypersensitivity reactions, including allergic reactions, exacerbation of asthma;
from the organ of vision: unknown - visual impairment;
on the part of the skin and its structures: infrequently - erythema, itching, rash; unknown - angioedema, urticaria, hair loss, abscess;
local reactions: unknown - reactions at the injection site.
The injection form of the drug contains lidocaine. In exceptional cases, adverse reactions characteristic of this component are possible:
from the digestive system: nausea, very rarely - vomiting;
from the nervous system: numbness of the tongue and lips, photophobia, diplopia, headache, confusion, muscle twitching, after use in high doses - tinnitus, agitation, anxiety, paresthesia, convulsions, loss of consciousness, coma, hyperacusis;
from the organs of vision: blurred vision, conjunctivitis; when used in high doses - nystagmus;
mental disorders: unknown - sleep disturbance;
from the cardiovascular system: arterial hypotension, transverse heart block; unknown - increased blood pressure; when used in high doses - arrhythmia, bradycardia, slowing of cardiac conduction, cardiac arrest, peripheral vasodilation, collapse, tachycardia, pain in the heart area;
from the immune system: suppression of the immune system, allergic reactions, including swelling, skin reactions, itching; very rarely - urticaria, hypersensitivity reactions, including anaphylactoid reactions (including anaphylactic shock), generalized exfoliative dermatitis;
from the respiratory system: depression or cessation of breathing, shortness of breath;
others: sensations of heat, cold or numbness of the extremities, malignant hyperthermia; when used in high doses - rhinitis;
local reactions: tingling of the skin at the injection site, abscess, slight burning sensation (disappears with the development of the anesthetic effect within 1 minute), thrombophlebitis.
Dona or Teraflex - which is better or more effective?
Manufacturer: BAYER PHARMA A.G.
(Germany) Release form: capsules
Active ingredient: chondroitin sulfate, glucosamine
Both drugs are in the group of stimulators of bone and cartilage tissue repair, have the same active substance glucosamine and restore their structure.
The Teraflex composition is enriched with a second active component, which makes it more effective. This combined property of the drug gives an anti-inflammatory and analgesic effect. This is equivalent to the use of analgesics and non-steroidal anti-inflammatory drugs. Teraflex will be the best for osteoarthritis with moderate pain.
If pain in the joint is severe, Dona injections will be more effective, as they quickly relieve acute pain.
Dona or Arthra - which is better?
Manufacturer: UNIFARM INC. (USA)
Release form: tablets
Active ingredient: glucosamine, chondroitin sulfate
Dona and Artra are used to treat joint diseases accompanied by a violation of the structure of cartilage tissue. Dona's analogue in tablets contains, in addition to glucosamine, chondroitin sulfate. The best drug for joints would be Artra, since the medication has more indications and fewer contraindications.
Both active ingredients enhance the action of each other, which leads to stimulation of the production of joint fluid and restoration of the damaged structure of synovial cartilage. The effectiveness of the analogue for osteoarthritis is confirmed by reviews from doctors who often prescribe Arthra for treatment.
Overdose
No cases of overdose have been reported. Based on acute and chronic toxicity studies in animals, toxic symptoms are unlikely to occur even at doses 200 times the therapeutic dose. however, with an overdose, the manifestations of adverse reactions may increase, so it is necessary to carry out symptomatic treatment aimed at restoring water and electrolyte balance.
The injection form of the drug contains the excipient lidocaine. The first symptoms of an overdose of lidocaine hydrochloride from the central nervous system may be numbness of the tongue and lips, agitation, anxiety, tinnitus, dizziness, blurred vision, tremor, depression, drowsiness, loss of consciousness, even coma, tonic-clonic convulsions. As indicated in the literature, cardiovascular and respiratory symptoms of overdose associated with lidocaine hydrochloride may include decreased blood pressure, collapse, AV block and respiratory depression. It is necessary to monitor the patient's cardiovascular and respiratory functions. A change in these parameters may indicate an overdose of the drug, so the patient should immediately be provided with access to oxygen. All complications require symptomatic treatment.
Dona or Sustilak - which is better?
Manufacturer: CELEBRITY BIOPHARMA LTD (India)
Release form: tablets
Active ingredient: glucosamine
Both products have the same active substance and mechanism of action on the cartilage tissue of the joints. Dona is a medicine that quickly relieves acute pain; it will be best for more pronounced destructive changes in the joint, preventing the patient from becoming disabled.
The therapeutic effect of using the Sustilak analogue manifests itself after a longer period of time. The drug can be used as a prophylaxis at the very initial manifestations of pathology and at any stage of development of joint destruction. In this case, Sustilak will be more effective in practical use.
Interactions
The use of mixtures containing ampoules of the drug with other injectable drugs should be avoided.
No specific drug interaction studies have been conducted. However, given the physicochemical and pharmacokinetic properties of glucosamine sulfate, a low potential for interactions can be assumed. In addition, it was found that glucosamine sulfate does not lead to either inhibition or increase in the activity of the main human CYP 450 enzymes.
In fact, the drug does not compete for absorption mechanisms; after absorption, it does not bind to blood plasma proteins, but is metabolized by inclusion as an endogenous substance in proteoglycans or is cleaved without the participation of enzymes of the cytochrome system, as a result of which it is unlikely to interact with other drugs.
However, an enhanced effect of coumarin anticoagulants has been reported in patients receiving concomitant treatment with glucosamine. In this regard, for such patients it is advisable to monitor coagulation parameters.
The drug is compatible with NSAIDs and GCS.
It is possible to enhance the gastrointestinal absorption of tetracycline.
The injection form of the drug contains the excipient lidocaine. Cimetidine, peptidine, bupivacaine, propranolol, quinidine, disopyramide, amitriptyline, nortriptyline, chlorpromazine, imipramine increase the level of lidocaine in the blood serum, reducing its hepatic metabolism. Norepinephrine has a synergistic effect when interacting with lidocaine.
MAO inhibitors should be used with caution, as they increase the risk of developing arterial hypotension and the local anesthetic effect of the latter is prolonged.
When used simultaneously with class IA antiarrhythmic drugs (including quinidine, procainamide, disopyramide), the QT interval is prolonged, and in very rare cases, AV block or ventricular fibrillation may develop.
The cardiotonic effect of the use of cardiac glycosides is weakened.
When used simultaneously with sedatives, the sedative effects are enhanced.
Phenytoin enhances the cardiodepressive effect of lidocaine.
When used simultaneously with procainamide, delusions and hallucinations are possible.
Lidocaine may enhance the effect of drugs that block neuromuscular transmission, since the latter reduce the conduction of nerve impulses.
Ethanol enhances the inhibitory effect of lidocaine on breathing.
Dona or Structum - which is better?
Manufacturer: PIERRE FABRE MEDICINE PRODUCTION (France)
Release form: capsules
Active ingredient: chondroitin sulfate
Dona and Structum are in the same pharmacological group, but act differently due to different active ingredients. Both drugs stimulate metabolism in cartilage tissue, slow down the progression of degenerative processes, and reduce joint pain.
Structum capsules will be more effective for osteoarthrosis and osteochondrosis of the intervertebral discs, as well as in the treatment of osteoarticular pathology. The drug is safer, can be prescribed to children over 15 years of age and has a minimum of contraindications. The results from its use last for a long time after the course of therapy.
Note!
Description of the drug Dona por. d/oral. sachet solution No. 20 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Dona or Rumalon injections - which is better?
Manufacturer: ROBAPHARM EOOD (Bulgaria)
Release form: solution for injection
Active ingredient: glycosaminoglycan-peptide complex
Rumalon, an analogue of Dona in ampoules, is a combined product of animal origin that stimulates the synthesis of collagen and mucopolysaccharides, slows down the destruction of cartilage and restores it. Both medications are chondroprotectors, but act through different mechanisms.
When comparing drugs for the treatment of osteoarticular pathology, the best remedy would be Rumalon, which has broader indications and fewer side effects. The medication is well tolerated and effective, as confirmed by patient reviews.
Contraindications
Hypersensitivity to glucosamine or any of the excipients; tendency to bleed. dona powder for oral solution - dysfunction of the liver and kidneys in the stage of decompensation.
Don's drug should not be used in patients with shellfish allergies, since the active substance is obtained from shellfish shells; such patients may be more prone to developing allergic reactions to glucosamine with a possible exacerbation of the symptoms of their disease.
Powder for oral solution contains aspartame and is therefore contraindicated in patients with phenylketonuria.
The injection form of the drug contains the excipient lidocaine, which has the following contraindications: cardiogenic shock, severe arterial hypotension, severe forms of chronic heart failure, reduced left ventricular function, AV block II-III degree, severe bradycardia, bleeding disorders, Wolf syndrome - Parkinson-White syndrome, Adams-Stokes syndrome, history of seizures caused by the use of lidocaine, sick sinus syndrome, severe liver dysfunction, hypovolemia, myasthenia gravis, injection site infections, hypersensitivity to other amide-type anesthetics (since there is an increased risk of developing cross-hypersensitivity reactions).
Elbona
Manufacturer: NPO FARMVILAR (Russia)
Release form: solution for injection, powder for the preparation of solution for oral administration
Active ingredient: glucosamine
Elbona belongs to the group of correctors that regulate the metabolism of bone and cartilage tissue. This analogue of Dona has an anti-inflammatory and analgesic effect, prevents the destruction of cartilage tissue and promotes its restoration. Acting on the joint capsule, the drug increases its permeability and increases the range of motion in the joint.
Chondrogard
Manufacturer: SOTEX CJSC (Russia)
Release form: solution for intramuscular and intraarticular administration
Active ingredient: chondroitin sulfate
Chondrogard is an analogue of Dona in injections. This is a high molecular weight mucopolysaccharide that has the properties to inhibit and reduce degenerative changes in cartilage tissue.
Under the influence of the medication, the inflammatory process of the synovial membrane of the joint is reduced, and phosphorus-calcium metabolism is restored. This process leads to a decrease in pain, relief of swelling and an increase in the range of active movements in the joint.