Mitral stenosis and insufficiency
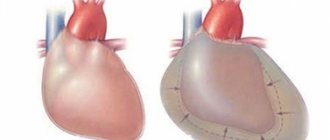
Stenosis of the left atrioventricular orifice (mitral valve stenosis) is one of the most common heart defects. Isolated mitral stenosis is observed in approximately half of patients with mitral defects. In other cases, there is a combined mitral defect - a combination of stenosis and mitral insufficiency, or a complex heart defect - a combination of mitral and aortic defects. The cause of the development of mitral valve stenosis is rheumatism (rheumatic endocarditis). Women get sick more often than men. Often the disease begins in childhood or adolescence and gradually progresses. In rheumatism, the inflammatory process affects the valve leaflets, the fibrous ring to which these leaflets are attached, and the tendon threads of the valve. Damage to the predominantly free edge of the leaflets and chords causes shortening of the leaflets, resulting in valve insufficiency. Predominant damage to the fibrous ring and the base of the valves leads to a narrowing of the opening, which makes it difficult to fill the left ventricle with blood during diastole. In healthy people, the area of the left atrioventricular opening is 4-6 cm. With “pure” mitral stenosis, the size of the opening is reduced to 1-1.5 cm. A decrease in the area of the mitral opening by 50% or more causes disturbances in cardiac hemodynamics. Difficulty in the passage of blood from the left atrium to the left ventricle during diastole is accompanied by stagnation and increased pressure in the left atrium. Since the pressure in the left ventricle does not change, a significant pressure difference (gradient) occurs between the high pressure in the atrium and the normal pressure in the ventricle. The more pronounced the mitral stenosis, the greater the diastolic pressure gradient. High pressure in the left atrium causes blood to flow from the atrium into the ventricle at high speed. This is also facilitated by the developing hypertrophy of the atrium, as a result of which the contraction of the atrium during its systole increases. However, an increase in pressure in the left atrium leads to stagnation in the pulmonary circulation. The pressure in the pulmonary artery reflexively increases, which leads to an increase in the load on the right side of the heart. A prolonged increase in pressure in the vessels of the small circle causes not only their spasm, but also sclerotic changes in the wall. If mitral stenosis itself is the first barrier to normal blood flow, then changes in the pulmonary vessels are the second barrier. Thus, the main burden in compensating for mitral stenosis falls on the weakest parts of the heart - first on the left atrium, then on the right half of the heart (right atria and ventricle). Symptoms. A patient with mitral stenosis often has a characteristic appearance - a combination of pale skin with a stagnant blush of the cheeks (mitral face). The patients themselves, especially if the defect arose in childhood, are somewhat behind in physical development and appear youthful. Often in these cases, a protrusion of the chest in the area of the heart (heart hump) is determined, caused by hypertrophy and dilatation of the right ventricle. A stream of blood passing through the narrowed mitral orifice and hitting the wall of the left ventricle causes it to oscillate. These vibrations are perceived when palpating the chest in the area of the apical impulse during diastole as a trembling of the chest. It is called "cat's purring", which is especially well detected when the patient is positioned on the left side, when the apex of the heart approaches the chest wall. Hypertrophy and dilatation of the right ventricle is accompanied by pulsation in the epigastric region. The size of the heart increases upward (due to the left atrium) and to the right (due to the right sections). Blood pressure with mitral valve stenosis either does not change, or there is a slight decrease in pulse pressure due to a decrease in systolic and an increase in diastolic pressure. The ECG reveals signs of hypertrophy of the left atrium and right ventricle; atrial extrasystole often occurs, which is later replaced by atrial fibrillation. The deposition of lime in the mitral valve leaflets in patients with mitral valve stenosis makes the leaflets inactive, and the amplitude of their oscillations decreases. A sharp narrowing of the mitral orifice and a weakening of the contractility of the left atrium myocardium lead to the fact that the diastolic murmur at the apex - the main symptom of stenosis - can no longer be detected. A picture of the so-called “silent stenosis” appears. Diastolic murmur is often detected in a very limited space at the apex of the heart, sometimes it is detected only after slight physical activity (for example, after squats) or with the patient positioned on the left side. Therefore, it may be necessary to re-register the FCG to clarify the presence of this noise. Diastolic murmur is associated with the flow of blood from the left atrium into the left ventricle during diastole. Since the pressure in the atrium at the beginning of diastole is maximum, the blood flow in this period occurs at maximum speed and, therefore, with maximum noise. Therefore, the murmur is protodiastolic in nature. As the atrium is emptied of blood, the pressure in it decreases, the rate of blood transfer to the ventricle decreases, and the intensity of the noise in the middle part of diastole also decreases. A new increase in murmur in the presystolic interval is caused by acceleration of blood flow during atrial systole. If a patient experiences atrial fibrillation, atrial systole does not occur and the presystolic murmur disappears. A significant increase in blood pressure in the pulmonary artery in patients with stenosis of the left atrioventricular orifice leads to expansion of the orifice of the vessel. The semilunar valves of the pulmonary artery become unable to completely close the opening during diastole, although the valves themselves remain intact. Relative (functional) insufficiency of the pulmonary valves occurs. This leads to the return of part of the blood at the beginning of diastole from the pulmonary artery to the right ventricle of the heart, which is accompanied by the appearance of a low-intensity murmur in the pulmonary artery in the second intercostal space at the left edge of the sternum during diastole (Trat-Still murmur). The progression of mitral stenosis is accompanied by an increase in pressure in the pulmonary circulation, which leads to hypertrophy and significant expansion of the cavity of the right ventricle. This leads to the occurrence of relative insufficiency of the tricuspid valve, when its leaflets, being unchanged, are unable to close the enlarged opening. In this case, during the systole of the right ventricle, part of the blood returns back to the right atrium. A systolic murmur occurs in the area of the tricuspid valve. Significant expansion of the right ventricle leads to the fact that the systolic murmur that occurs with tricuspid valve insufficiency is recorded not only in the fourth-fifth intercostal space at the right edge of the sternum, but also over the entire surface of the heart, including in the apex. There is difficulty in differentiating the murmur of tricuspid valve insufficiency from the systolic murmur of mitral insufficiency. To differentiate noise, use the fact that the noise that occurs with tricuspid valve insufficiency is not carried out to the left axillary region, where the noise is carried out with mitral insufficiency. With tricuspid valve insufficiency, the systolic murmur increases at the height of inspiration as a result of increased blood flow to the right side of the heart; with mitral insufficiency, the murmur weakens during inspiration. The course of mitral stenosis is often accompanied by a number of severe complications. Stagnation and increased pressure in the pulmonary circulation are accompanied by hemoptysis, shortness of breath, and attacks of cardiac asthma. Dystrophic and sclerotic changes in the atria stretched with blood cause the appearance of atrial fibrillation. In this case, blood clots form in the atria, which can cause embolism in various organs (brain, kidneys, vessels of the extremities, etc.), with the development of a clinical picture characteristic of this pathology (convulsions, paralysis, hematururia, etc.). The prognosis for mitral stenosis is determined by the severity of rheumatism, the frequency of rheumatic attacks, the degree of narrowing of the mitral orifice and the condition of the myocardium. If the course is favorable, the disease can last for many years. Repeated rheumatic attacks, progressive narrowing of the mitral orifice and deterioration of myocardial function, quickly lead the patient to disability, and severe complications lead to sudden death.
General information
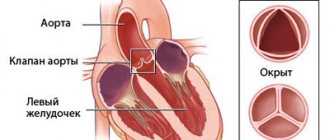
Mitral stenosis is an acquired monovalvular defect that impairs the activity of the heart muscle as a result of morphological and/or functional changes in the structure of the mitral valve.
Normally, it has a bicuspid (bicuspid) structure and is located on the border of the left atrium and the left ventricle, preventing the reverse flow (regurgitation) of blood into the chamber of the left atrium in systole, that is, during ventricular contraction. However, infectious lesions, inflammation, autoimmune reactions, overload, and enlargement of the heart chambers can cause pathological changes in the valve system in the form of narrowing of the atrioventricular orifice, which leads to disruption of intracardiac hemodynamics and systemic blood flow. The disease is characterized by a slow course - the first symptoms may appear after 40-50 years, although the formation of pathological narrowing usually begins at a young age. A downward change in the diameter of the atrioventricular orifice occurs in approximately 0.05-0.08% of the population, more often in females.
Methods for diagnosing heart defects
In order to establish a diagnosis of heart disease, an anamnesis is collected, the presence of diseases that could lead to deformation of the heart valve is revealed: rheumatic diseases, infectious, inflammatory processes, autoimmune diseases, injuries.
- The patient must be examined to identify the presence of shortness of breath, cyanosis, edema, and pulsation of peripheral veins. Using percussion, the boundaries of the heart are identified and sounds and murmurs in the heart are listened to. The size of the liver and spleen is determined.
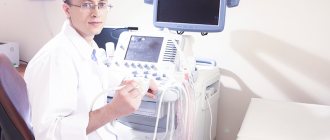
- The main method for diagnosing valve pathology is echocardiography
, which allows you to identify the defect, determine the area of the opening between the atrium and the ventricle, the size of the valves, cardiac fraction, pressure in the pulmonary artery.
More accurate information about the condition of the valves can be obtained by performing transesophageal echocardiography. - Electrocardiography is also used in diagnostics, which allows one to assess the presence of atrial and ventricular hypertrophy and identify signs of overload of the heart. Daily Holter ECG monitoring allows you to identify rhythm and conduction disturbances.
- Highly informative methods for diagnosing heart defects are cardiac MRI or cardiac MSCT. Computed tomography scans provide precise and numerous sections, which can be used to accurately diagnose the defect and its type.
- Laboratory tests play an important role in diagnosis, including urine and blood tests, determination of blood sugar, cholesterol levels, and rheumatoid tests. Laboratory tests allow us to identify the cause of the disease, which plays an important role in subsequent treatment and patient behavior.
Classification
Taking into account the state of general hemodynamics, mitral stenosis is divided into compensated, subcompensated and decompensated. In addition, valve defects can differ depending on the etiology and be rheumatic (the most common type - in 50-80% of cases), atherosclerotic, syphilitic, as well as the outcome of bacterial endocarditis .
The most important factor in dividing mitral stenosis into types is the severity of the defect and its degree of influence on intracardiac hemodynamics. Violations can be moderate and moderately severe with a transmitral gradient of 1 – 25 mm Hg, and also not exceed 5 mm Hg. st and does not have any effect on the state of intracardiac hemodynamics.
Classification of mitral stenosis by severity
Mitral stenosis is usually isolated, but can also be combined with disorders of other valves or against the background of mitral valve insufficiency.
Causes
Most often, mitral stenosis develops as a result of previously suffered rheumatism , in other cases the cause becomes:
- infective endocarditis ;
- chronic valvulitis ;
- carcinoid syndrome;
- mitral valve calcification
- thrombus;
- obstruction during the tumor process and the development of myxoma ;
- heart injuries.
Congenital anomaly of the mitral valve in the direction of narrowing of the atrioventricular orifice is extremely rare, for example, with Lutembasch syndrome .