Doctors and patients speak differently about the drug neotigazon for acute and chronic psoriasis.
Some patients noted an immediate improvement in their well-being, some took longer to recover, and others did not feel any effect.
Let's look at the main properties and characteristics of the drug and read reviews from real doctors and buyers.
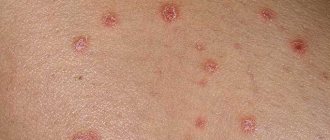
Psoriasis is a chronic non-infectious disease, accompanied by the appearance of reddish-pink rashes on the skin. A person with psoriasis experiences itching and flaking. A dermatological problem is accompanied by the fact that skin cells in the affected areas divide tens of times faster than in normal conditions.
Their inability to fully mature provokes the loss of intercellular contacts, which leads to the appearance of scales. The immune system is also subject to this pathology, resulting in an inflammatory reaction at the site of the lesion.
Action and effect of the drug
Retinoids react with proteins of the nucleus and cytoplasm at the cellular level. The latter elements take part in the proliferation of epithelial cells, influencing keratinization and controlling the thickness of the epidermis (upper layer).
Thus, neotigazon helps maintain a normal level of frequency of skin cell division, helps regulate the thickness of the epidermis and prevents the formation of “extra” cells.
Release form
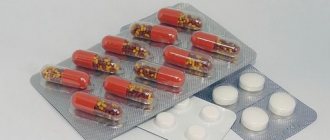
The product is available in capsules of 30, 50 and 100 pieces.
Dosage
The effectiveness of treatment with neotigazon is determined by its dosage. Pharmacies offer the product in capsules of 25 and 10 mg. It is recommended to use the product with meals; be sure to take the tablet with milk, unsweetened tea or pure water.
For adults
A strict dose is prescribed by the attending physician, who objectively assesses the general condition of the patient, his age, the presence/absence of diseases, and so on. First, the minimum dosage is prescribed; if there is no effect of treatment, it may be increased.
If the main active ingredient and auxiliary components of the drug are tolerated by the patient without complications, the course is extended for 2 months. The maximum dose per day is 75 mg.
For children
The drug is also used in childhood; the dosage is prescribed by the doctor based on weight and age. Usually, 0.5 mg of acitretin per kg of body weight (daily dose) is sufficient.
The amount of the drug may be increased if there are special complications during the disease.
Pharmacokinetics of the drug
Acitretin is an aromatic synthetic substance, otherwise known as vitamin A. The effect of the substance is to normalize the proliferation and differentiation of epidermal cells.
The effectiveness of the drug and the timing of final elimination have not been clinically established. The maximum content of the active substance in the blood was established 2 hours after taking the drug.
The highest bioavailability is during meals (about 60%), with fluctuations from 36% to 95%. Acitretin easily penetrates tissue and binds to proteins up to 99%. May pass into breast milk in large quantities; the drug is strictly contraindicated during lactation.
Side effects
They appear in 90% of all patients taking the drug, but disappear after reducing the dosage or discontinuing the use of capsules. At the beginning of the treatment process, the main symptoms may worsen.
Hypervitaminosis may occur - excess vitamin A in the body leads to excessive dryness of the lips, which can be eliminated by using a rich cream. The table shows the prevalence of side effects from the drug neotigazon.
Nervous system: side effects
- Headache;
- dizziness;
- increase in pressure.
Organs of vision
- Inflammatory reactions of the mucous membrane;
- Conjunctivitis;
- Contact lens intolerance;
- Hemeralopia;
- Ulcer in the cornea area.
Hearing
Based on the feedback provided by users of this drug, the following side effects in the field of hearing can be identified.
- The occurrence of tinnitus;
- Problems with hearing function.
Vascular system
- Feeling of a “rush” of blood to the face.
Breath
- Rhinitis;
- Bleeding from the nose.
Gastrointestinal tract
- Dry mouth;
- Feeling thirsty;
- Stomatitis;
- Severe abdominal pain;
- Flatulence;
- Vomiting and nausea;
- Diarrhea;
- Problems with taste;
- Gingivitis.
Liver
- Hepatitis;
- Jaundice.
Skin tissue
- Itching;
- Severe peeling of the skin;
- Fragility of the skin;
- Excessive stickiness;
- Change in hair structure;
- Dermatitis;
- Brittle nails;
- Formation of cracks;
- Erythema;
- Granuloma.
Musculoskeletal tissue
- Bone pain.
Changes in childhood
After prolonged treatment with the drug, significant changes in bone tissue were found in children, including premature closure of the epiphysis and skeletal hyperostosis.
Neotigason®
Neotigazone should only be prescribed by physicians who have experience in the use of systemic retinoids and understand the risk of teratogenicity of acitretin.
Women of childbearing age should not drink alcohol during treatment with Neotigazon, since there is clinical evidence that etretinate can be formed in the body when taking acitretin and alcohol simultaneously. The mechanism of this metabolic transformation has not been established, so it is unclear whether other substances may also be involved. Ethanol should be avoided for 2 months after discontinuation of acitretin therapy.
Women of childbearing age should not receive blood transfusions from patients receiving Neotigazon. Therefore, during treatment with Neotigazon and for a year after its completion, blood donation is prohibited.
Liver function should be monitored before starting treatment with Neotigazoyoma, every 1-2 weeks during the first month after starting treatment, and then every 3 months. If test results indicate pathology, monitoring should be carried out weekly. If liver function does not normalize or worsens further, Neotigazon should be discontinued. In this case, it is recommended to continue monitoring liver function for at least another 3 months.
It is necessary to monitor fasting serum cholesterol and triglyceride levels, especially in patients at risk (lipid metabolism disorders, diabetes mellitus, obesity, alcoholism) and during long-term treatment.
In patients with diabetes, retinoids may improve or worsen glucose tolerance, so blood glucose levels should be checked more frequently than usual early in treatment.
Adults receiving long-term therapy with Neotigazon should undergo regular examinations, taking into account the possibility of ossification abnormalities (see "Side effects"). If such disorders occur, the issue of continuing treatment should be discussed with the patient, carefully weighing the possible risks and benefits of using the drug.
In children, growth parameters and bone development should be closely monitored.
Due to the possibility of impaired night vision, patients should be warned about the need to exercise caution when driving a car or working with machines and mechanisms at night. Visual impairment should be closely monitored.
Currently, not all the consequences of using Neotigazon that may occur throughout life are known.
Cost of the drug
Depending on the place of purchase, the price may vary. The average cost is 900 (for 30 pieces) of 10 mg. If you need to buy 30 capsules of 25 mg of active ingredient, the price is 2600 rubles.
Drug interactions
- Concomitant use of vitamin A with other retinoids should be avoided.
- Neotigazon should not be used with tetracyclines: this can lead to increased blood pressure.
- Combining the drug with etretinate - Tigasone - can lead to hepatitis.
- When used in one course with phenytoin, the drug helps reduce the binding of the first substance to proteins.
- During the course of treatment, contraceptives such as the minipill may not be effective.
- It is strictly forbidden to use tablets together with ethyl alcohol.
Since the half-life of the drug is 120 days, the use of contraceptives is recommended for two years after its use.
Neotigason®
Neotigazone should only be prescribed by physicians who have experience in the use of systemic retinoids and understand the risk of teratogenicity of acitretin.
The doctor must provide all patients, both men and women, with complete information about the teratogenic effect of Neotigazon and measures to prevent pregnancy.
Women of childbearing age during treatment with Neotigazon and for 2 months after stopping treatment should not drink alcohol, as well as alcohol-containing drinks, foods and medications, since there is clinical evidence that when taking acitretin and alcohol simultaneously, the formation of etretinate. Etretinate is highly teratogenic and has a longer T1/2 (approximately 120 hours) compared to acitretin. Pregnancy protection measures and a pregnancy test must be performed within 2 years after completion of treatment with Neotigazon (see section Use during pregnancy and breastfeeding).
Women of childbearing age should not receive blood transfusions from patients receiving Neotigazon. Therefore, during treatment with Neotigazon and for two years after its completion, blood donation is prohibited.
Due to the risk of developing congenital pathologies of the fetus, the drug should not be transferred to other patients. Unused or expired medication should be destroyed in accordance with current legislation.
Liver function should be monitored before starting treatment with Neotigazon, every 1-2 weeks for the first two months after starting treatment, and then every 3 months. If test results indicate pathology, monitoring should be carried out weekly. If liver function does not normalize or worsens further, Neotigazon should be discontinued. In this case, it is recommended to continue monitoring liver function for at least another 3 months.
It is necessary to monitor fasting serum cholesterol and triglyceride levels, especially in patients at risk (lipid metabolism disorders, diabetes mellitus, obesity, alcoholism) before starting treatment, within a month after starting treatment, and then every 3 months.
Visual impairment should be closely monitored.
In rare cases, benign intracranial hypertension has been reported. If severe headaches, nausea, vomiting and blurred vision occur, Neotigazon should be immediately discontinued and the patient referred to a neurologist.
Adults, especially elderly patients receiving long-term therapy with Neotigazon, should undergo regular examinations, taking into account the possibility of ossification abnormalities (see section Side effects). If these complications occur, subsequent treatment should be discussed with the patient based on a careful assessment of the benefit-risk ratio.
In children, growth parameters and bone development should be closely monitored. Currently, not all the consequences of using Neotigazon are known, which may occur throughout life with long-term use of the drug Neotigazon.
The effect of ultraviolet (UV) radiation on the body is enhanced when taking retinoids. Patients should avoid excessive exposure to sunlight and limit the use of UV lamps (eg, solarium treatments).
If necessary, sunscreens with a high protection factor (at least SPF 15) should be used. Treatment with high doses of retinoids may cause mood changes, including irritability, aggression, and depression.
Patient risk groups:
In patients with diabetes mellitus, alcoholism, obesity, with risk factors for the cardiovascular system or lipid metabolism disorders taking Neotigason, it is necessary to monitor cholesterol levels and/or glucose concentrations more often or monitor symptoms indicating the possible development of complications from the cardiovascular system (for example, measure blood pressure).
In patients with diabetes, retinoids may improve or worsen glucose tolerance, so blood glucose levels should be checked more frequently than usual early in treatment.
For all patients at risk in whom the observed cardiovascular complications do not normalize or worsen further, the dose of Neotigason should be reduced or a decision should be made to discontinue it.
The drug contains maltodextrin, which contains glucose, so Neotigazon should not be used by patients with a rare hereditary disease - glucose-galactose malabsorption.
Based on the available data on the degree of influence on the incidence of birth defects when fertilizing women with sperm and seminal fluid of male patients taking acitretin, it can be concluded that there is a minimal risk of developing teratogenic effects.
Reviews from doctors
Anna Yakusheva, dermatologist, work experience – 15 years
Neotigazon is a broad-spectrum drug. At the end of the treatment course, most of my patients began to improve, the spots from psoriasis “went away”, and their general health improved. I prescribe the drug only in extreme cases, when treatment with other means seems ineffective. I categorically do not recommend taking the drug without a doctor’s recommendation (prescription), in higher dosages, and especially during pregnancy, since in these cases the side effects are most pronounced.
Irina Dobrovolskaya, dermatologist, work experience – 8 years
My patients use neotigazon. But I prescribe the prescription only in extreme cases, because I know that the product has many side effects. The drug is effective if it is used strictly according to the prescription and strictly in compliance with the dosage; in all other situations it will be harmful and even dangerous. The drug is not prescribed to girls and women of childbearing age; other means of getting rid of rashes and itching are being sought.
Olga Ivanova, dermatologist, 23 years of experience
Psoriasis is a serious problem that requires surgical treatment. The disease itself is not harmful or dangerous, but its consequences can ruin patients’ lives and interfere with normal life activities. Modern developments of drugs for psoriasis include many progressive drugs, reviews from my colleagues and patients about them are very different. One such remedy is neotigazon.
The drug is sold in capsule form and is aimed at suppressing the symptoms of the disease - skin itching, peeling and redness. In 90% of cases, patients get rid of symptoms and no longer complain about the manifestations of the disease. I recommend using the product only in situations where the use of other drugs - ointments, creams and lotions - is inappropriate, as well as in acute forms of the disease. This is due to a large number of side effects from the use of the medicine.
My patients complained of severe dizziness. Symptoms may worsen at the initial stage of using the drug. The use of the drug also causes diarrhea, vomiting, and nausea. If the doctor begins to talk about this drug to the patient, he should ask the latter about the presence of chronic diseases and characteristics of the body. I also do not recommend taking the pills to girls of childbearing age, pregnant or lactating women.
results
Within 8 weeks, a significant positive effect from the therapy was achieved: erythema and infiltration resolved, fissures epithelialized, dryness and flaking of the skin in the lesions decreased, and quality of life normalized (Fig. 3, 4).
Rice. 3. Condition of the palms after 8 weeks of secukinumab therapy.
Rice. 4. Condition of the feet after 8 weeks of secukinumab therapy. The patient tolerates the treatment well. Changes in dermatological indices during therapy are presented in the table.
Dynamics of the main assessment indices (BSA, PASI, PGA, DLQI) during secukinumab therapy in patient S.
The patient continues to receive secukinumab therapy at a dose of 300 mg monthly subcutaneously for 9 months (14 injections). No side effects or adverse events were identified throughout the entire treatment period. The patient is very pleased with the result. Currently, the patient lives a full life, is employed (he did not work before treatment), and has no complaints.