Home | About us | Delivery | Advertisers | Login | Registration
The pharmacy is closed on Sundays and holidays.
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
Buy Betadine vaginal suppositories 200 mg No. 7 in pharmacies
Betadine Buy Betadine in pharmacies
DOSAGE FORMS vaginal suppositories 200 mg
MANUFACTURERS Egis Pharmaceutical Works SA (Hungary) Egis Pharmaceutical Plant OJSC (Hungary)
GROUP Antiseptics - halogens and halogen-containing products
COMPOSITION The active substance is povidone-iodine.
INTERNATIONAL NON-PROPENTED NAME Povidone-Iodine
SYNONYMS Aquazan, Brownodine B. Brown, Iodine-Ka, Iodovidone, Iodoxide, Iodosept, Povidone-Iodine, Polyiodine
PHARMACOLOGICAL ACTION Antiseptic, disinfectant. It has a wide spectrum of antimicrobial action (gram-positive and gram-negative bacteria - with the exception of M/ tuberculosis, fungi, viruses, protozoa). It has a longer action than inorganic iodine solution. Suppositories are made on a water-soluble basis and do not have an irritating effect. When applied topically, there is virtually no absorption of iodine from the skin, mucous membrane or wounds.
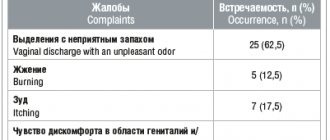
INDICATIONS FOR USE Solution for external use 10%. Treatment and prevention of wound infections in surgery, traumatology, combustiology, dentistry. Treatment of bacterial, fungal and viral skin infections, prevention of superinfection in dermatological practice. Treatment of bedsores, trophic ulcers, diabetic foot. Disinfection of the skin and mucous membranes of patients in preparation for surgical interventions, invasive studies (punctures, biopsies, injections, etc.) Disinfection of the skin around catheters, drainages, probes. Disinfection of the oral cavity during dental operations. Disinfection of the birth canal during “minor” gynecological operations (artificial termination of pregnancy, insertion of an intrauterine device, coagulation of erosion or polyp, etc.). Foaming solution 7.5% for external use. "Disinfectant bathing", for complete or partial treatment of patients before surgery. Disinfection of the skin of the hands of surgical personnel. Hygienic treatment of patients. Hygienic treatment of hands when in contact with infected patients. Processing of non-metallic instruments and patient care items. Concentrate for preparing a solution for topical use. Inflammatory processes in the mouth and throat. Condition after surgical interventions in the mouth and throat. Eliminating bad breath. Ointment. Bacterial and fungal skin infections, burns, trophic ulcers, bedsores, infectious dermatitis, abrasions, wounds. Vaginal suppositories. Treatment of vaginitis (mixed, nonspecific), candidiasis, trichomoniasis, genital herpes; preoperative preparation.
CONTRAINDICATIONS Hypersensitivity, thyroid adenoma, hyperthyroidism, Dühring's dermatitis herpetiformis, chronic renal failure, simultaneous use of radioactive iodine, pregnancy, lactation, children under 8 years of age.
SIDE EFFECTS When used on a large wound surface and mucous membranes, systemic reabsorption of iodine may occur, which may affect tests of the functional activity of the thyroid gland, and also lead to the development of neutropenia. In some cases, with hypersensitivity to the drug, local manifestations of allergic reactions to iodine (hyperemia, burning, itching, swelling, pain) may occur, requiring discontinuation of the drug. Long-term use (more than 7-10 days) can cause the phenomenon of iodism (“metallic” taste in the mouth, increased salivation, swelling of the eyes or larynx, etc.), if this occurs, you should stop using the drug and consult a doctor.
INTERACTION Incompatible with other disinfectants and antiseptics, especially those containing alkalis, enzymes and mercury. Activity decreases in an acidic environment.
METHOD OF APPLICATION AND DOSAGE 1 suppository 1-2 times a day; Duration of treatment is 7-14 days.
OVERDOSE No information available.
SPECIAL INSTRUCTIONS Solution 10%, foaming solution 7.5%. It is necessary to ensure that no excess solution remains under the patient. Should not be heated before use. Concentrate 8.5% for preparing a solution for topical use. It has a deodorizing effect - it eliminates bad breath that occurs after eating food, alcoholic beverages, and tobacco. Solutions 10, 7.5%; concentrate 8.5%; ointment 10%. The presence of pus and blood can reduce the antimicrobial effect of the drug. A colored film is formed at the site of application, which persists until the entire amount of active iodine is released, which means that the effect of the drug ceases. Should not be used for bites from insects, domestic or wild animals. All dosage forms. Coloring on leather and fabrics is easily washed off with water. Avoid contact of the drug with the eyes.
STORAGE CONDITIONS At a temperature of 0-25°C, in a place protected from light.
Modern Pharmaceuticals of Russia
POVIDONE-IODINE, vaginal suppositories
Trade name: Povidone-Iodine International non-proprietary or generic name:
Povidone-iodine
Dosage form:
vaginal suppositories
Description:
Suppositories are brown to dark brown in color, torpedo-shaped.
Marbling of the surface is allowed. ATC code:
G01AX11
Pharmacotherapeutic group:
Antiseptic.
Pharmacodynamics:
Antiseptic drug.
Blocks amino groups of cellular proteins. Has a wide spectrum of antimicrobial action. Active against gram-positive and gram-negative bacteria (including Escherichia coli, Staphylococcus aureus), mycoplasma, chlamydia, fungi (for example, the genus Candida), viruses, protozoa. The active substance of the drug, iodine, is in the form of a complex with polyvinylpyrrolidone (povidone), which belongs to iodophors that bind iodine. The concentration of active iodine is 0.1-1%. In combination with polyvinylpyrrolidone, iodine significantly loses its local irritating effect and is therefore well tolerated when applied to mucous membranes, skin and wound surfaces. Upon contact with the skin and mucous membranes, iodine is gradually and evenly released, exerting a bactericidal effect on microorganisms. Remains at the site of application. Pharmacokinetics
Absorption and distribution
When povidone-iodine comes into contact with the mucous membrane, iodine may be absorbed and its content in the blood may increase (the concentration returns to its original value 7-14 days after the last use of the drug). In patients with normal thyroid function, increased iodine absorption does not cause significant changes in thyroid hormonal function.
Removal
Iodine is excreted primarily by the kidneys.
The half-life (T1/2) after intravaginal use is about 48 hours. Indications for use:
Acute and chronic infectious and inflammatory diseases of the vagina (Trichomonas, fungal, viral, nonspecific, mixed infections); bacterial vaginitis.
Prevention of infectious and inflammatory complications before gynecological interventions (artificial termination of pregnancy, installation and removal of an intrauterine device, diathermocoagulation of the cervix, hysterosalpingography and others).
Contraindications:
Hypersensitivity to iodine and other components of the drug, thyrotoxicosis (hyperthyroidism), Dühring's dermatitis herpetiformis, thyroid adenoma, simultaneous use of radioactive iodine, children under 8 years of age.
With caution:
Chronic renal failure, pregnancy, breastfeeding, old age.
Use during pregnancy and breastfeeding:
Iodine penetrates the placental barrier and is excreted in breast milk.
The use of the drug is not recommended from the second trimester of pregnancy and during breastfeeding. The use of the drug during these periods is possible only if the expected benefit to the mother outweighs the potential risk to the fetus and child. If it is necessary to use the drug in these cases, treatment is possible under individual medical supervision. In newborns and infants whose mothers used POVIDONE-IODINE, it is necessary to monitor thyroid function. Directions for use and dosage:
Intravaginally. Having previously freed the suppository from the contour cellular packaging, it is inserted, lying on the back, deep into the vagina.
For acute vaginitis - 1 suppository 2 times a day for 7 days, for subacute and chronic vaginitis - 1 suppository 1 time a day before bed for 14 days (regardless of the phase of the menstrual cycle).
For preoperative prevention of complications of infectious origin in gynecology, the drug is administered intravaginally 2 times a day (morning and evening) for 1 to 7 days. Side effects:
Hypersensitivity reactions to the drug at the site of application: hyperemia, itching. Allergic reactions are possible, including contact dermatitis with the formation of psoriasis-like red small bullous elements. If such phenomena occur, use of the drug should be discontinued.
If any of the side effects indicated in the instructions get worse or you notice any other side effects not listed in the instructions, tell your doctor!
Overdose
Symptoms
Acute iodine intoxication is characterized by: a metallic taste in the mouth, increased salivation, a burning sensation or pain in the mouth or throat; irritation and swelling of the eyes; skin reactions; gastrointestinal disorders and diarrhea; renal dysfunction and anuria; circulatory failure; laryngeal edema with secondary asphyxia, pulmonary edema, metabolic acidosis, hypernatremia.
Treatment
Symptomatic therapy and maintenance of the function of vital organs and systems (including electrolyte balance, kidney and thyroid function).
Interaction with other drugs:
Povidone-iodine is incompatible with oxidizing agents, alkali salts and acidic substances.
It is not recommended to use povidone-iodine simultaneously with hydrogen peroxide and products containing mercury, silver and enzymes. Special instructions:
In the presence of blood, the bactericidal effect of povidone-iodine may be reduced.
In the case of non-manifest hyperthyroidism and other diseases of the thyroid gland (especially in elderly patients), the drug is used only according to strict indications and under constant medical supervision.
Due to the oxidizing properties of povidone-iodine, trace amounts may cause false-positive results in some types of tests to detect occult blood in the stool and blood or glucose in the urine.
During use of povidone-iodine, iodine uptake by the thyroid gland may be decreased, which may affect the results of some diagnostic tests (eg, thyroid scintigraphy, protein-bound iodine, radioactive iodine measurements), and there may be an interaction with iodine preparations used for the treatment of thyroid diseases. To obtain undistorted results of thyroid scintigraphy after long-term therapy with povidone-iodine, it is recommended to maintain a sufficiently long period of time without this drug. If symptoms of hyperthyroidism occur during treatment, thyroid function should be checked. Caution should be exercised when regularly using Iodasept in patients with previously diagnosed renal failure.
Regular use of the drug should be avoided in patients receiving lithium preparations.
Caution is recommended when administering vaginal suppositories to virgins.
Suppositories have a spermicidal effect, and therefore are not recommended for use in persons planning pregnancy.
It is recommended to use sanitary pads when using suppositories.
After contact with the drug, avoid contact with the eyes.
Effect on the ability to drive vehicles and machinery:
Does not affect the ability to drive a vehicle or other machinery.
Release form:
5 suppositories each in a blister pack made of PVC/PE polymer film.
One or two contour cell packages with five suppositories are placed together with instructions for medical use of the drug in a pack of boxed cardboard grade A or chrome-ersatz.
Storage conditions:
In a place protected from light at a temperature not exceeding 25 ° C.
Keep out of the reach of children! Shelf life:
3 years.
Do not use after the expiration date stated on the packaging.
Dispensing conditions:
Without a prescription.