Ammonia
(hydrogen nitride) is a chemical compound of nitrogen and hydrogen with the formula NH3; under normal conditions, it is a colorless gas with a sharp, characteristic odor.
The density of ammonia is almost half that of air, MPC.z. 20 mg/m3 - IV hazard class (low-hazardous substances) according to GOST 12.1.007. The solubility of NH3 in water is extremely high - about 1200 volumes (at 0 °C) or 700 volumes (at 20 °C) per volume of water. In refrigeration technology it is called R717, where R is Refrigerant (refrigerant), 7 is the type of refrigerant (inorganic compound), 17 is the molecular weight.
Ammonia is one of the most important products of the chemical industry; its annual global production exceeds 180 million tons.
The ammonia molecule is shaped like a triangular pyramid with a nitrogen atom at the top. Three unpaired p-electrons of the nitrogen atom participate in the formation of polar covalent bonds with the 1s-electrons of three hydrogen atoms (N - H bonds), the fourth pair of outer electrons is lone, it can form a covalent bond via a donor-acceptor mechanism with a hydrogen ion, forming an ion ammonium NH4+. The non-bonding two-electron cloud is strictly oriented in space, so the ammonia molecule has high polarity, which leads to its good solubility in water.
In liquid ammonia, the molecules are connected to each other by hydrogen bonds. A comparison of the physical properties of liquid ammonia with water shows that ammonia has lower boiling points (tboiling −33.35 °C) and melting points (tmelting −77.70 °C), as well as lower density and viscosity (7 times less than the viscosity of water ), conductivity (almost does not conduct electric current) and dielectric constant. This is to some extent explained by the fact that the strength of hydrogen bonds in liquid ammonia is significantly lower than that of water; and also by the fact that an ammonia molecule has only one pair of unshared electrons, in contrast to two pairs in a water molecule, which does not make it possible to form a branched network of hydrogen bonds between several molecules. Ammonia easily transforms into a colorless liquid with a density of 681.4 kg/m³, which strongly refracts light. Like water, liquid ammonia is highly associated, mainly through the formation of hydrogen bonds. Liquid ammonia is a good solvent for a very large number of organic, as well as many inorganic compounds. Solid ammonia is cubic crystals.
Content
- 1 Chemical properties
- 2 History
- 3 Origin of the name
- 4 Liquid ammonia
- 5 Complexation
- 6 Biological role
- 7 Physiological action
- 8 Application
- 9 Receipt 9.1 Consumption rates per ton of ammonia
Chemical properties
- Due to the presence of a lone electron pair, in many reactions ammonia acts as a Bronsted base or a complexing agent (the concepts of “nucleophile” and “Bronsted base” should not be confused. Nucleophilicity is determined by the affinity for a positively charged particle. A base has an affinity for a proton. The concept of “base” is a special case concept of "nucleophile"). So, it adds a proton, forming an ammonium ion:
NH3 + H+ ⟶ NH4+.
- An aqueous solution of ammonia (“ammonia”) has a slightly alkaline reaction due to the process:
NH3 + H2O ⟶ NH4+ + OH− , Ko=1.8⋅10−5.
- Interacting with acids, it gives the corresponding ammonium salts:
NH3 + HNO3 ⟶ NH4NO3.
- Ammonia is also a very weak acid (10,000,000,000 times weaker than water) and is capable of forming salts with metals - amides, imides and nitrides. Compounds containing NH2− ions are called amides, NH2− are called imides, and N3− are called nitrides. Amides of alkali metals are prepared by treating them with ammonia:
2NH3 + 2K ⟶ 2KNH2 + H2.
Amides, imides and nitrides of a number of metals are formed as a result of certain reactions in liquid ammonia. Nitrides can be produced by heating metals in a nitrogen atmosphere.
Metal amides are analogues of hydroxides. This analogy is strengthened by the fact that the OH− and NH2− ions, as well as the H2O and NH3 molecules, are isoelectronic. Amides are stronger bases than hydroxides, and therefore undergo irreversible hydrolysis in aqueous solutions:
NaNH2 + H2O ⟶ NaOH + NH3.
and in alcohols:
KNH2 + C2H5OH ⟶ C2H5OK + NH3.
Like aqueous solutions of alkalis, ammonia solutions of amides conduct electric current well, which is due to dissociation:
KNH2 ⇄ K+ + NH2− .
Phenolphthalein in these solutions turns crimson; when acids are added, they are neutralized. The solubility of amides changes in the same sequence as the solubility of hydroxides: LiNH2 - insoluble, NaNH2 - slightly soluble, KNH2, RbNH2 and CsNH2 - highly soluble.
- When heated, ammonia decomposes and exhibits reducing properties. So, it burns in an oxygen atmosphere, forming water and nitrogen. The oxidation of ammonia with air on a platinum catalyst produces nitrogen oxides, which are used industrially to produce nitric acid:
2NH3 →1200-1300∘C N2 + 3H2 (reaction is reversible), 4NH3 + 3O2 ⟶ 2N2 + 6H2O (without a catalyst, at elevated temperature), 4NH3 + 5O2 ⟶ 4NO + 6H2O (in the presence of a catalyst, at elevated temperature).
The reducing ability of NH3 is the basis for the use of ammonia NH4Cl for cleaning the metal surface from oxides during soldering:
3CuO + 2NH4Cl ⟶ 3Cu + 3H2O + 2HCl + N2.
By oxidizing ammonia with sodium hypochlorite in the presence of gelatin, hydrazine is obtained:
2NH3 + NaOCl ⟶ N2H4 + NaCl + H2O.
- Halogens (chlorine, iodine) form dangerous explosives with ammonia - nitrogen halides (nitrogen chloride, nitrogen iodide).
- Ammonia reacts with halogenated alkanes through nucleophilic addition, forming a substituted ammonium ion (method for producing amines):
NH3 + CH3Cl ⟶ [CH3NH3]Cl (methyl ammonium hydrochloride).
- It produces amides with carboxylic acids, their anhydrides, acid halides, esters and other derivatives. With aldehydes and ketones - Schiff bases, which can be reduced to the corresponding amines (reductive amination).
- At 1000 °C, ammonia reacts with coal, forming hydrocyanic acid HCN and partially decomposing into nitrogen and hydrogen. It can also react with methane, forming the same hydrocyanic acid:
2CH4 + 2NH3 + 3O2 ⟶ 2HCN + 6H2O,
- NH4OH ⟶ NH3 + H2O.
- Forms complex ammonia salts with copper salts and silver
Cu(NO3)2 + 4NH3 ⟶ [Cu(NH3)4](NO3)2, Cu3(PO4)2 + 12NH3 ⟶ [Cu(NH3)4]3(PO4)2, Cu(CH3COO)2 + 4NH3 ⟶ [ Cu(NH3)4](CH3COO)2, AgNO3 + 2NH3 ⟶ [Ag(NH3)2]NO3.
The ammonia synthesis column built in 1921 by BASF in Oppau is now located at the University of Karlsruhe.
Consequences of poisoning
After ammonia intoxication, a person can face very serious irreversible consequences. First of all, the central nervous system suffers, which entails a number of complications:
- The brain ceases to fully perform its functions and begins to malfunction, because of this, intelligence decreases, mental illness, amnesia, and nervous tics appear.
- The sensitivity of some parts of the body decreases.
- The functioning of the vestibular apparatus is disrupted. Because of this, a person feels constant dizziness.
- The hearing organs begin to lose their functionality, which leads to deafness.
- When the eye covers are damaged, vision and its acuity are reduced; in the worst case, the victim will experience blindness.
- The onset of death. It depends on how high the gas concentration in the air was and how much ammonia vapor entered the body.
Knowing and following the prescribed safety measures means protecting yourself from the risk of a threat to your own life or a worse fate - disability, loss of hearing or vision.
Story
Ammonia was first isolated in its pure form by J. Priestley in 1774, who called it “alkaline air.” Eleven years later, in 1785, C. Berthollet established the exact chemical composition of ammonia. Since that time, research has begun around the world on producing ammonia from nitrogen and hydrogen. Ammonia was very necessary for the synthesis of nitrogen compounds, since their production from Chilean saltpeter was limited by the gradual depletion of the latter's reserves. The problem of decreasing nitrate reserves became more acute towards the end of the 19th century. Only at the beginning of the 20th century was it possible to invent a process for the synthesis of ammonia suitable for industry. This was carried out by F. Haber, who began working on this problem in 1904 and by 1909 had created a small contact apparatus in which he used increased pressure (in accordance with Le Chatelier’s principle) and an osmium catalyst. On July 2, 1909, Haber tested the apparatus in the presence of K. Bosch and A. Mittasch, both from the Baden Aniline and Soda Works (BASF), and obtained ammonia. By 1911, K. Bosch had created a large-scale version of the apparatus for BASF, and then the world's first ammonia synthesis plant was built and put into operation on September 9, 1913, which was located in Oppau (now a district within the city of Ludwigshafen am Rhein) and belonged to BASF. In 1918, F. Haber won the Nobel Prize in Chemistry “for the synthesis of ammonia from its constituent elements.” In Russia and the USSR, the first batch of synthetic ammonia was produced in 1928 at the Chernorechensky Chemical Plant.
Methods of obtaining
Ammonia is produced by the interaction of hydrogen and nitrogen molecules in industry. The production technique is called the Haber process. The entire reaction proceeds with the release of heat and a decrease in volume. Thus, the reaction is carried out at reduced ambient temperature and elevated pressure. The balance shifts to the right. Under these conditions, the reaction rate is low, and as the temperature readings increase, the rate begins to increase. To carry out the reaction safely, special equipment is required to maintain elevated pressure.
To accelerate the achievement of an equilibrium state, catalyst materials are used - iron with a porous composition and a certain percentage of additives.
In all respects, the process of producing ammonia occurs at a temperature of 500 degrees Celsius and in the presence of high pressure, reaching 350 atmospheres. The percentage of production if these factors are met will be 30 percent. In industry, the process is circular. The composition is cooled and ammonia is removed, and unreacted nitrogen and hydrogen are returned for re-synthesis. This method of ammonia extraction in industry is considered the most economical.
In laboratory conditions, ammonia is produced through the action of alkalis. The chemical element is obtained by heating ammonium with lime. To dry ammonia, use lime and soda.
Due to the physical and chemical properties of ammonia, it is successfully used in industry, manufacturing, medicine, chemistry and many other areas of human activity.
origin of name
Ammonia (in European languages its name sounds like “ammoniac”) owes its name to the Ammon oasis in North Africa, located at the crossroads of caravan routes. In hot climates, urea (NH2)2CO, contained in animal waste products, decomposes especially quickly. One of the decomposition products is ammonia. According to other sources, ammonia got its name from the ancient Egyptian word amonian
. This was the name given to people who worshiped the god Amun. During their rituals, they sniffed the mineral ammonia (NH4Cl), which, when heated, evaporates ammonia.
Item Description
In the liquid form of ammonia, the molecules are connected by hydrogen bonds. The temperature, viscosity and density of ammonia are significantly lower compared to water. The boiling process starts at 33 degrees, and the burning or melting process starts at 77 degrees Celsius. The conductivity and dielectric constant of ammonia are low. Consequently, the bond strength in the liquid state is low.
Similar to water, ammonia associates in its liquid state due to the presence of hydrogen bonding. The transition of the chemical composition to the state of a colorless liquid with a density of 681 kilograms per cubic meter is rapid. There is practically no current conductivity in this state.
Liquid ammonia
Liquid ammonia, although to a small extent, dissociates into ions (autoprotolysis), which shows its similarity to water:
2NH3 ⟶ NH4+ + NH2−.
The self-ionization constant of liquid ammonia at −50 °C is approximately 10−33 (mol/l)².
Liquid ammonia, like water, is a strong ionizing solvent in which a number of active metals dissolve: alkali, alkaline earth, Mg, Al, as well as Eu and Yb. Unlike water, these metals do not react with liquid ammonia, but dissolve and can be released in their original form upon evaporation of the solvent. The solubility of alkali metals in liquid NH3 is several tens of percent. Some intermetallic compounds containing alkali metals, for example, Na4Pb9, are also dissolved in liquid ammonia NH3.
Dilute solutions of metals in liquid ammonia are colored blue, concentrated solutions have a metallic sheen and look like bronze. When ammonia evaporates, alkali metals are released in pure form, and alkaline earth metals are released in the form of complexes with ammonia [E(NH3)6], which have metallic conductivity. When heated slightly, these complexes decompose into metal and NH3.
The metal dissolved in NH3 gradually reacts to form an amide:
2Na + 2NH3 ⟶ 2NaNH2 + H2.
The metal amides resulting from the reaction with ammonia contain a negative ion, NH2−, which is also formed during the self-ionization of ammonia. Thus, metal amides are analogues of hydroxides. The reaction rate increases when going from Li to Cs. The reaction is significantly accelerated in the presence of even small H2O impurities.
Metal-ammonia solutions have metallic conductivity; in them, metal atoms decompose into positive ions and solvated electrons surrounded by NH3 molecules. Metal-ammonia solutions, which contain free electrons, are the strongest reducing agents.
Aggregate states
Ammonia can be in different states of aggregation:
- It is present as a colorless gas with an unpleasant, pungent odor under normal conditions.
- It can also dissolve very well in water, so it can be stored in the form of an aqueous solution with a certain concentration. It liquefies and becomes a liquid as a result of pressure and extreme cooling.
- Ammonia has a solid state in which it appears as colorless cubic crystals.
Complexation
Due to their electron-donating properties, NH3 molecules can enter complex compounds as ligands. Thus, the introduction of excess ammonia into solutions of d-metal salts leads to the formation of their amino complexes:
CuSO4 + 4NH3 ⟶ [Cu(NH3)4]SO4. Ni(NO3)2 + 6NH3 ⟶ [Ni(NH3)6](NO3)2.
Complexation is usually accompanied by a change in the color of the solution. Thus, in the first reaction, the blue color (CuSO4) turns into dark blue (the color of the complex), and in the second reaction the color changes from green (Ni(NO3)2) to blue-violet. The strongest complexes with NH3 are formed by chromium and cobalt in the +3 oxidation state.
Prevention in case of poisoning
First aid in this case consists of several simple steps. First, you need to take the victim out into the fresh air and wash his face and eyes with running water. Even those who were not very good at chemistry know from school: alkali is neutralized by acid, so the mouth and nose must be rinsed with water with the addition of lemon juice or vinegar.
If the poisoned person has lost consciousness, you should lay him on his side in case of vomiting, and if his pulse and breathing stop, give him a cardiac massage and artificial respiration.
Biological role
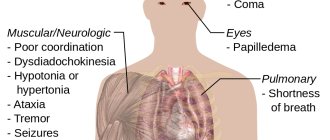
The main symptoms of hyperammonemia (increased levels of endogenous ammonia to toxic concentrations).
Ammonia is an important source of nitrogen for living organisms. Despite the high content of free nitrogen in the atmosphere (more than 75%), very few living creatures are able to use the free, neutral diatomic nitrogen of the atmosphere, N2 gas. Therefore, to include atmospheric nitrogen into biological circulation, in particular into the synthesis of amino acids and nucleotides, a process called “nitrogen fixation” is necessary. Some plants depend on the availability of ammonia and other nitrogen compounds formed in the soil as a result of the decomposition of organic (plant and animal) residues. Others, such as legumes, take advantage of a symbiosis with nitrogen-fixing bacteria (rhizobia), which are able to synthesize ammonia from atmospheric nitrogen using enzymes called nitrogenases. Although it is unlikely that biomimetic methods will ever be developed that can compete in productivity with chemical methods for producing ammonia from nitrogen, scientists are nevertheless making great efforts to better understand the mechanisms of biological nitrogen fixation. Scientific interest in this problem is partly motivated by the unusual structure of the active catalytic site of the nitrogen-fixing enzyme (nitrogenase), which contains an unusual bimetallic molecular assembly Fe7MoS9.
Ammonia is also an end by-product of amino acid metabolism, namely the product of deamination catalyzed by enzymes such as glutamate dehydrogenase. Excretion of unchanged ammonia is a common route for ammonia detoxification in aquatic creatures (fish, aquatic invertebrates, and some amphibians). In mammals, including humans, ammonia is usually quickly converted to urea, which is much less toxic and, in particular, less alkaline and less reactive as a reducing agent. Urea is the main component of urine solids. Most birds, reptiles, insects, and arachnids, however, emit uric acid rather than urea as the main nitrogen residue.
Ammonia also plays an important role in both normal and pathological animal physiology. Ammonia is produced during normal amino acid metabolism, but is highly toxic in high concentrations. Animal livers convert ammonia to urea through a series of sequential reactions known as the urea cycle. Impaired liver function, such as that seen in cirrhosis, can impair the liver's ability to detoxify ammonia and convert it into urea, resulting in increased levels of ammonia in the blood, a condition called hyperammonemia. A similar result - an increase in the level of free ammonia in the blood and the development of hyperammonemia - is caused by the presence of congenital genetic defects in urea cycle enzymes, such as ornithine carbamyltransferase. The same result can be caused by a violation of the excretory function of the kidneys in severe renal failure and uremia: due to a delay in the release of urea, its level in the blood increases so much that the “urea cycle” begins to work “in the opposite direction” - excess urea is hydrolyzed back by the kidneys into ammonia and carbon dioxide gas, and as a result, the level of ammonia in the blood increases. Hyperammonemia contributes to disturbances of consciousness and the development of soporous and comatose states in hepatic encephalopathy and uremia, as well as to the development of neurological disorders often observed in patients with congenital defects of urea cycle enzymes or organic acidurias.
Less pronounced, but clinically significant, hyperammonemia can be observed in any process in which increased protein catabolism is observed, for example, with extensive burns, tissue compression or crush syndrome, extensive purulent-necrotic processes, gangrene of the extremities, sepsis, etc., and also for some endocrine disorders, such as diabetes mellitus, severe thyrotoxicosis. The likelihood of hyperammonemia occurring in these pathological conditions is especially high in cases where the pathological condition, in addition to increased protein catabolism, also causes a pronounced impairment of the detoxifying function of the liver or the excretory function of the kidneys.
Ammonia is important for maintaining normal acid-base balance in the blood. After ammonia is formed from glutamine, alpha-ketoglutarate can be further broken down to form two bicarbonate molecules, which can then be used as a buffer to neutralize dietary acids. The ammonia obtained from glutamine is then excreted in the urine (both directly and in the form of urea), which, taking into account the formation of two bicarbonate molecules from ketoglutarate, results in a total loss of acids and a shift in blood pH to the alkaline side. In addition, ammonia can diffuse through the renal tubules, combine with and be excreted with hydrogen ion (NH3 + H+ ⟶ NH4+), and thereby further promote the elimination of acids from the body.
Ammonia and ammonium ions are a toxic byproduct of metabolism in animals. In fish and aquatic invertebrates, ammonia is released directly into the water. In mammals (including aquatic mammals), amphibians, and sharks, ammonia is converted to urea in the urea cycle because urea is much less toxic, less chemically reactive, and can be more efficiently “stored” in the body until it can be excreted. In birds and reptiles, ammonia produced during metabolism is converted to uric acid, which is a solid residue and can be excreted with minimal loss of water.
Symptoms of poisoning
Below are a number of signs of ammonia poisoning:
- Severe cough, difficulty breathing.
- Burning in the eyes, tearing, painful reaction to bright light.
- Burning in the mouth and nasopharynx.
- Dizziness, headache.
- Abdominal pain, vomiting.
- Reduced hearing threshold.
- With more serious poisoning, the following are possible: loss of consciousness, convulsions, respiratory arrest, acute heart failure. The combination of violations can lead the victim to a coma.
Physiological action
Ammonia is toxic. According to its physiological effect on the body, it belongs to the group of substances with asphyxiating and neurotropic effects, which, if inhaled, can cause toxic pulmonary edema and severe damage to the nervous system. Ammonia has both local and resorptive effects.
Ammonia vapors strongly irritate the mucous membranes of the eyes and respiratory organs, as well as the skin. This is what a person perceives as a pungent odor. Ammonia vapors cause excessive lacrimation, eye pain, chemical burns of the conjunctiva and cornea, loss of vision, coughing attacks, redness and itching of the skin. When liquefied ammonia and its solutions come into contact with the skin, a burning sensation occurs, and a chemical burn with blisters and ulcerations is possible. In addition, liquefied ammonia absorbs heat when it evaporates, and when it comes into contact with the skin, frostbite of varying degrees occurs. The smell of ammonia is felt at a concentration of 37 mg/m³.
The maximum permissible concentration in the air of the working area of the production premises (MPCr.z.) is 20 mg/m³. In the atmospheric air of populated areas and in residential premises, the average daily concentration of ammonia (MPC.s.) should not exceed 0.04 mg/m³. The maximum single concentration in the atmosphere is 0.2 mg/m³. Thus, the smell of ammonia indicates that the permissible limits have been exceeded.
Irritation of the pharynx occurs when the ammonia content in the air is 280 mg/m³, eyes - 490 mg/m³. When exposed to very high concentrations, ammonia causes skin damage: 7-14 g/m³ - erythematous, 21 g/m³ or more - bullous dermatitis. Toxic pulmonary edema develops when exposed to ammonia for an hour at a concentration of 1.5 g/m³. Short-term exposure to ammonia at a concentration of 3.5 g/m³ or more quickly leads to the development of general toxic effects.
In the world, the maximum concentration of ammonia in the atmosphere (more than 1 mg/m³) is observed in the Indo-Gangetic Plain, in the Central Valley of the USA and in the Turkestan (formerly South Kazakhstan) region of Kazakhstan.
Areas of use
The scope of application of ammonia and ammonia alcohol is wide; it is used in almost all spheres of human activity, from technological processes to medicine and household needs.
Application of ammonia
Ammonia is widely used as a refrigerant in various household and industrial equipment.
It is one of the most important products used in the chemical industry . In particular, it is used in production:
- ammonia;
- additives in building materials for use in frosty conditions;
- polymers, soda and nitric acid;
- fertilizers;
- explosives.
Using ammonia alcohol
Ammonia alcohol is used in medicine and in everyday life.
Medical use is indicated in the following cases:
- to stimulate breathing in cases of fainting: to do this, bring a bottle of ammonia to the victim’s nose; the bottle should be kept 5 cm from the nose to avoid burns;
- to stimulate vomiting in various types of poisoning: 10 drops per glass of water and give to drink;
- externally for local cooling for pain from neuralgia or myositis;
- externally for itching from insect bites: make a solution of alcohol and water in a ratio of 1 part alcohol to 10 parts water and wipe the itchy area;
- externally for treating surgeon's hands as an antiseptic;
- orally for alcohol poisoning: 5 drops of alcohol per 1 glass of water. This allows you to quickly wake up, collect your thoughts and adequately answer questions;
- as an antiseptic for sterilizing needles in the absence of an autoclave or disposable needles;
- to remove warts: moisten a cotton swab with undiluted alcohol and apply to the wart for 5-6 seconds, without touching healthy skin to avoid burns;
- in dentistry in a certain concentration when treating the oral cavity;
- and in other cases.