Nosological classification (ICD-10)
- D65-D69 Bleeding disorders, purpura and other hemorrhagic conditions
- H35 Other retinal diseases
- H35.6 Retinal hemorrhage
- H43.1 Vitreous hemorrhage
- I78.8 Other diseases of capillaries
- I79.2 Peripheral angiopathy in diseases classified elsewhere
- N92 Heavy, frequent and irregular menstruation
- N93 Other abnormal bleeding from the uterus and vagina
- R58 Bleeding, not elsewhere classified
- Z100* CLASS XXII Surgical practice
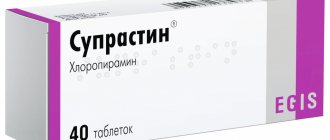
Dicynon solution for intravenous and intramuscular administration, 125 mg/ml amp. 2ml No. 50
Indications
Prevention and stopping of bleeding: parenchymal and capillary bleeding (including traumatic, in surgery during operations on highly vascularized organs and tissues, during surgical interventions in dental, urological, ophthalmological, otolaryngological practice, intestinal, renal, pulmonary bleeding, metro- and menorrhagia with fibroids and others), secondary bleeding due to thrombocytopenia and thrombocytopathy, hypocoagulation, hematuria, intracranial hemorrhage (including in newborns and premature infants), nosebleeds due to arterial hypertension, bleeding caused by taking medications, hemorrhagic diathesis (including Werlhoff disease, von Willebrand-Jurgens disease, thrombocytopathy), diabetic microangiopathy (hemorrhagic diabetic retinopathy, repeated retinal hemorrhage, hemophthalmos).
pharmachologic effect
A hemostatic agent, it also has angioprotective and proaggregant effects. Stimulates the formation of platelets and their release from the bone marrow. The hemostatic effect, caused by the activation of thromboplastin formation at the site of damage to small vessels and the decrease in the formation of prostacyclin PgI2 in the vascular endothelium, promotes increased adhesion and aggregation of platelets, which ultimately leads to stopping or reducing bleeding. Increases the rate of formation of the primary thrombus and enhances its retraction, has virtually no effect on the concentration of fibrinogen and prothrombin time. Doses of more than 2-10 mg/kg do not lead to a greater effect. With repeated administrations, thrombus formation increases. Possessing antihyaluronidase activity and stabilizing ascorbic acid, it prevents the destruction and promotes the formation of mucopolysaccharides with high molecular weight in the capillary wall, increases the resistance of capillaries, reduces their fragility, and normalizes permeability in pathological processes. Reduces fluid leakage and diapedesis of blood cells from the vascular bed, improves microcirculation. Does not have a vasoconstrictor effect. Restores pathologically altered bleeding time.
It does not affect the normal parameters of the hemostatic system. The hemostatic effect with intravenous administration of etamsylate occurs within 5-15 minutes, the maximum effect appears after 1-2 hours. The effect continues for 4-6 hours, then gradually weakens over 24 hours; with intramuscular administration, the effect occurs within 30-60 minutes.
Drug interactions
Administration of ethamsylate at a dose of 10 mg/kg 1 hour before the administration of dextrin solutions with an average molecular weight of 30,000-40,000 prevents the antiaggregation effect of the latter; administration of ethamsylate after dextran solutions does not have a hemostatic effect.
A combination with aminocaproic acid and menadione sodium bisulfite is possible.
Etamsylate is pharmaceutically incompatible (in the same syringe) with other drugs.
Dosage regimen
Used orally, intramuscularly, intravenously, locally, subconjunctivally, retrobulbarly. The dosage regimen is set individually, depending on the indications, clinical situation, patient's age, and dosage form used.
Contraindications for use
Hypersensitivity to etamsylate, acute porphyria, hemoblastosis in children, thrombosis, thromboembolism; pregnancy, lactation (breastfeeding); children under 3 years of age (for oral administration).
Carefully
History of thrombosis, thromboembolism; bleeding due to an overdose of anticoagulants; liver and/or kidney dysfunction.
Use in children
It can be used in children according to indications, in doses, regimens and dosage forms recommended according to age. It is necessary to strictly follow the instructions in the instructions for etamsylate preparations regarding contraindications for the use of specific dosage forms of etamsylate in children of different ages.
Restrictions for children
Use with caution
Use in elderly patients
Use with caution in elderly patients to avoid the risk of exacerbation of chronic diseases.
Restrictions for elderly patients
Use with caution
Use for liver dysfunction
Use with caution in patients with impaired liver function.
Restrictions for liver dysfunction
Use with caution
Use during pregnancy and breastfeeding
Contraindicated for use during pregnancy and breastfeeding.
Restrictions when breastfeeding
Contraindicated
Restrictions during pregnancy
Contraindicated
Use for renal impairment
Use with caution in patients with impaired renal function.
Restrictions for impaired renal function
Use with caution
special instructions
Before starting treatment, other causes of bleeding should be excluded.
Etamsylate is not effective in patients with a low platelet count.
For hemorrhagic complications associated with an overdose of anticoagulants, it is recommended to use specific antidotes.
The use of etamsylate in patients with impaired blood coagulation system parameters is possible, but it should be supplemented by the administration of drugs that eliminate the identified deficiency or defect of coagulation factors.
Due to the increased risk of a pronounced decrease in blood pressure with parenteral administration of etamsylate, caution should be exercised in patients with unstable blood pressure or a tendency to hypotension.
Side effect
From the digestive system:
often - nausea, diarrhea, heaviness in the epigastric region.
For the skin and subcutaneous tissues:
often - skin rash; frequency unknown - facial skin flushing.
From the nervous system:
often - headache; frequency unknown - dizziness, paresthesia of the lower extremities.
From the cardiovascular system:
very rarely - thromboembolism, marked decrease in blood pressure.
From the hematopoietic system:
very rarely - agranulocytosis, neutropenia, thrombocytopenia.
From the musculoskeletal system:
very rarely - arthralgia.
Other:
often - asthenia; very rarely - allergic reactions, fever.
Possible product names
- Dicynon solution for intravenous and intramuscular administration, 125 mg/ml amp. 2ml No. 50
- DICINON 250 MG AMP IV V/M 2 ML No. 50
- DICYNON 0.125/ML 2ML N50 AMP R-R V/V V/M
- DICYNON RR IV, IM INJECTED 125 MG/ML 2 ML. X50
- DICYNON SOLUTION FOR INJECTION. FOR IV AND IM ADMINISTRATION 250 MG AMP 2 ML No. 50
- DICYNONE 125MG/ML SOLUTION FOR IV AND IM INSTRUCTION. 2ML AMP. X50 B M (R)
- (Dicynone) Dicynone solution for IV and IM administration, 125 mg/ml amp. 2ml No. 50
Directions for use and doses
IM, IV (slow).
The optimal daily dose of etamsylate for adults is 10–20 mg/kg/day, divided into 3–4 doses.
For adults. During surgical interventions, 250–500 mg is administered prophylactically intravenously or intramuscularly 1 hour before surgery; during surgery, 250–500 mg (1–2 amp.) is administered intravenously; if necessary, this dose can be repeated again . After surgery, 250–500 mg (1–2 amps) is administered every 4–6 hours until the risk of bleeding disappears.
To stop bleeding, 250–500 mg (1–2 amps) are administered intravenously or intramuscularly, followed by 250 mg every 4–6 hours for 5–10 days.
In the treatment of metro- and menorrhagia, the drug is used in a single dose of 250 mg IV or IM every 6-8 hours for 5-10 days.
For diabetic microangiopathy, the drug is administered subconjunctivally or retrobulbarly at a dose of 125 mg (1/2 amp.).
The drug Dicynon® can be used topically (for example, in the case of a skin graft, after tooth extraction, etc.): a sterile swab or napkin is soaked in the solution and applied to the wound.
For children. The daily dose is 10–15 mg/kg, divided into 3–4 doses.
In neonatology, Dicynon® is administered intramuscularly or intravenously at a dosage of 10 mg/kg (0.1 ml = 12.5 mg). Treatment should begin within the first 2 hours after birth, then every 6 hours for 4 days.
If Dicynone® is mixed with saline, it should be used immediately.
Content
- Characteristics of dicynon (amp. 125 mg/ml 2 ml No. 10)
Composition: Active ingredient - Etamsylate.
Pharmacological Action: Hemostatic, angioprotective.
Stimulates the formation of platelets and their release from the bone marrow.
Moderately accelerates the formation of tissue thromboplastin.
It is well absorbed both intramuscularly and orally.
The effective concentration in the blood is 0.05-0.02 mg/ml.
It is evenly distributed in various organs and tissues (depending on the degree of their blood supply).
Weakly binds to proteins and formed elements of blood.
It is quickly eliminated from the body, mostly unchanged.
5 minutes after intravenous administration, 20-30% of the administered amount is excreted by the kidneys; it is completely eliminated from the body after 4 hours.
Acts on the platelet link of hemostasis, without having a pronounced effect on the coagulation link.
Possessing antihyaluronidase activity and stabilizing ascorbic acid, it prevents the breakdown of mucopolysaccharides of the vascular wall (increases the resistance of capillaries, reduces their permeability and fragility).
Increases the rate of formation of the primary thrombus and enhances its retraction, with virtually no effect on the level of fibrinogen and the prothrombin index.
The most acceptable doses are 2-10 mg/kg (in this range the effect is proportional to the dose), further increasing them only slightly increases the effect.
With repeated administrations, thrombus formation increases.
After a course of treatment, the effect lasts 5-8 days, gradually weakening.
With intravenous administration, activation of hemostasis begins after 5-15 minutes, reaches a maximum after 1-2 hours, remains at a sufficient level for 4-6 hours, gradually weakening and stopping by the end of the day.
Indications for Use: Parenchymal and capillary bleeding, secondary bleeding due to thrombocytopenia and thrombocytopathy, prevention of intra- and postoperative bleeding (during operations on blood vessels and highly vascularized tissue), diabetic microangiopathy, nosebleeds with arterial hypertension, hemorrhagic syndrome while taking ascorbic acid and indirect anticoagulants.
Method of Use: Prevention of bleeding during surgical operations - 1-2 ampoules IV or IM 1 hour before surgery, 1-2 ampoules IV during surgery; 2-4 ampoules in the next 24 hours after surgery.
To stop bleeding, IV or IM - 1-2 ampoules, then every 4-6 hours - 1 ampoule.
Diabetic microangiopathy - 1 ampoule IM 2 times a day for 2-3 months.
Interaction: Pharmaceutically incompatible (in the same syringe) with others.
drugs.
Administration at a dose of 10 mg/kg 1 hour before rheopolyglucin prevents the antiaggregation effect of the latter (administration after rheopolyglucin does not have a hemostatic effect).
Combination with aminocaproic acid, vikasol, and anticoagulants is acceptable.
Reduces the severity of hemorrhagic syndrome caused by acetylsalicylic acid and indirect anticoagulants.
Side Effects: Heartburn, feeling of heaviness in the epigastric region, facial flushing, paresthesia in the lower extremities, decreased blood pressure, slight dizziness, headache.
Contraindications: Hypersensitivity, hemorrhages due to anticoagulants, thrombosis, thromboembolism.
Overdose: No data available.
Special Instructions: No data available.