Pharmacological properties
Pharmacodynamics.
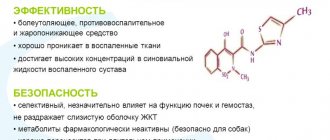
Meloxicam belongs to the oxicam group of NSAIDs, a selective COX-2 inhibitor that contains enolic acid. the drug has a pronounced anti-inflammatory, analgesic and antipyretic effect. The mechanism of action of the drug is the ability to inhibit the biosynthesis of prostaglandins - mediators of inflammation due to the selective inhibition of tsog-2. Clinical studies have revealed significantly less toxicity of meloxicam compared to other drugs (for example, naproxen, piroxicam and diclofenac), which, by inhibiting cog-1 and cog-2 to the same extent, can damage the gastrointestinal tract and kidneys. the selectivity coefficient ic50 of cog-1/cog-2 for meloxicam is 2, thanks to this the drug exhibits the necessary therapeutic effect and, compared with non-selective cog inhibitors, is less likely to cause adverse reactions from the gastrointestinal tract and kidneys. Meloxicam does not affect platelet aggregation and bleeding time. Pharmacokinetics. Suction. Meloxicam is well absorbed from the gastrointestinal tract regardless of food intake. The bioavailability of the drug is 89%, Cmax in blood plasma is achieved after 5–6 hours and, depending on the dose taken, is 0.4–1 mg/ml after taking 7.5 mg and 0.8–2.0 mg/ml after taking 15 mg of the drug. An equivalent concentration is achieved on the 3rd–5th day of treatment. Long-term use of the drug (more than 1 year) does not cause an increase in its concentration in the blood plasma compared to the levels at the beginning of use.
Distribution. About 99.5% of the drug binds to blood plasma proteins. The volume of distribution averages 11 liters. Meloxicam penetrates into the synovial fluid, where its concentration is approximately 2 times lower than in blood plasma.
Metabolism. Biotransformation occurs in the liver by oxidation of methyl groups with the formation of 4 inactive metabolites.
Elimination. About 43% of the dose is excreted in the urine, the rest in bile. 5% of the dose is excreted unchanged in bile. T½ of the drug is 20 hours.
Hepatic and renal failure do not have a significant effect on the pharmacokinetics of meloxicam. Plasma clearance is 8 ml/min and decreases in the elderly.
Application
In adults, Melbek is used once a day with a small amount of liquid.
For osteoarthritis: 5 mg/day; if necessary, the dose can be increased to 15 mg/day.
For rheumatoid arthritis and ankylosing spondylitis: 15 mg 1 time per day; depending on the therapeutic effect, the dose can be reduced to 7.5 mg 1 time per day.
For patients at high risk of side effects and patients on dialysis, treatment should begin with a dose of 7.5 mg once daily.
The maximum daily dose of meloxicam for adults is 15 mg.
The duration of treatment is determined individually.
Side effects
Possible abdominal pain, constipation, flatulence, diarrhea; anemia, itching, dizziness, headache, peripheral edema.
Rarely, transient increases in liver function tests (increased transferase activity or plasma bilirubin concentration), esophagitis, peptic ulcer or gastrointestinal bleeding may occur; leukopenia and thrombocytopenia; stomatitis, urticaria, tinnitus, drowsiness; increased blood pressure, facial erythema with a feeling of heat, tachycardia; changes in kidney function parameters (increased levels of creatinine and/or urea in the blood plasma). When taking a drug with a potential negative effect on bone marrow cells (especially methotrexate), pancytopenia may develop.
Very rarely - perforation, hepatitis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis, asthma attack that occurs as a result of taking acetylsalicylic acid, acute renal failure, conjunctivitis, visual impairment, angioedema, anaphylactoid/anaphylactic reactions.
Side effects:
When using the drug Melbek, the following may develop:
- nausea, vomiting, constipation, diarrhea, abdominal pain, flatulence, belching, esophagitis, gastroduodenal ulcers, hepatitis, colitis, gastritis, transient increases in bilirubin or transaminases; - anemia, changes in blood count (leukopenia, thrombocytopenia, cytopenia); - skin irritation, itching, urticaria, stomatitis; - dizziness, tinnitus, headache, mood changes, lethargy; - swelling, hot flashes, increased blood pressure, palpitations; - increased levels of urea, creatinine, acute renal failure; - visual impairment, conjunctivitis; - hypersensitivity reaction, angioedema.
special instructions
It is necessary to strictly monitor the use of the drug in patients with a history of asthma. The drug should be used with caution in conditions of dehydration, debilitated patients, the elderly, patients with heart failure, as well as those taking anticoagulant and antiplatelet drugs.
Like other NSAIDs, meloxicam may infrequently cause interstitial nephritis, glomerulonephritis, renal papillary necrosis, or nephrotic syndrome. Such complications can occur in patients with chronic renal failure, after extensive surgery (which caused hypovolemia), as well as in patients with cirrhosis of the liver.
If symptoms of gastrointestinal bleeding, skin changes, or gingivitis or conjunctivitis occur, you should immediately stop using the drug.
In patients with minor or moderately severe renal impairment (creatinine clearance 25 ml/min), the dose of the drug does not need to be reduced.
Impact on the ability to drive vehicles and operate other machinery. There is no data regarding the effect of the drug on the ability to drive vehicles or operate complex machinery. If dizziness or drowsiness occurs, you must avoid those activities that require psychomotor activity.
Melbek tablets 15 mg No. 10x2
Name
Melbek tablet 15 mg in bl. in pack №10x2
Main active ingredient
Meloxicam
Release form
tablet
Compound
1 tablet contains: meloxicam 7.5 mg (or 15 mg). Excipients: crospovidone (Kollidon Cl), povidone (PVP K30, polyvinylpyrrolidone), microcrystalline cellulose PH 102, sodium citrate, anhydrous lactose, anhydrous colloidal silicon (Aerosil 200), magnesium stearate.
Description
7.5 mg tablets: light yellow, round, chamfered tablets with a score line on one side. 15 mg tablets: light yellow round tablets with a cross-score on one side.
Dosage
7.5 mg or 15 mg
Indications for use
Short-term symptomatic therapy for exacerbation of osteoarthritis. Long-term symptomatic treatment of rheumatoid arthritis or ankylosing spondylitis.
Contraindications
hypersensitivity to meloxicam or any of the excipients, as well as other non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin. For patients who have developed symptoms of bronchial asthma, nasal polyps, angioedema or urticaria after taking aspirin or other NSAIDs, taking meloxicam is contraindicated; history of gastrointestinal bleeding or perforation due to previous treatment with nonsteroidal anti-inflammatory drugs (NSAIDs); acute or previous stomach ulcer or gastrointestinal bleeding (at least 2 episodes of confirmed ulcer or bleeding); gastrointestinal bleeding, a history of cerebrovascular bleeding, or other conditions with an increased risk of bleeding; nonspecific inflammatory bowel diseases in the acute phase (Crohn's disease, ulcerative colitis); severe liver failure; patients with severe renal failure who are not on hemodialysis; severe heart failure; contraindicated for the treatment of intraoperative pain during coronary artery bypass grafting (CABG); period of pregnancy and breastfeeding; children and adolescents under 16 years of age.
Use during pregnancy and lactation
Melbek is contraindicated during pregnancy. Inhibition of prostaglandin synthesis may have undesirable effects on pregnancy and fetal development. Data from epidemiological studies indicate an increased risk of spontaneous abortion, heart defects and gastroschisis in the fetus after the use of prostaglandin synthesis inhibitors in early pregnancy. The absolute risk of developing cardiovascular defects increased from less than 1% to 1.5%. This risk increases with increasing dose and duration of therapy. In the third trimester of pregnancy, the use of any prostaglandin synthesis inhibitors can lead to the following developmental disorders of the fetus: premature closure of the ductus arteriosus and pulmonary hypertension due to toxic effects on the cardiopulmonary system; renal dysfunction, with further development of renal failure with oligohydroamniosis. During labor, the mother may experience an increase in the duration of bleeding (and the antiaggregation effect can develop even at a low dosage) and a decrease in uterine contractility, and, as a result, an increase in the duration of labor. Despite the lack of data on experience with the drug Melbek, it is known that NSAIDs pass into breast milk. Therefore, these drugs are contraindicated during lactation. The use of meloxicam, like other drugs that block cyclooxygenase/prostaglandin synthesis, may affect fertility, so this drug is not recommended for women planning pregnancy. If the ability to conceive in women is impaired or when undergoing examination for infertility, it is necessary to consider discontinuing meloxicam. Effect on the ability to drive a car and use machinery. Studies have not been conducted to assess the effect of the drug on the ability to drive a car or use machinery. However, patients should be warned that adverse reactions such as visual disturbances, dizziness, drowsiness and other disorders of the central nervous system may occur. Patients with the above symptoms should avoid performing potentially dangerous activities, such as driving or operating machinery.
Directions for use and doses
The daily dose should be taken once during meals with water or other liquid. Adverse reactions can be reduced by administering the lowest effective dose for the shortest possible time necessary to control symptoms. The need for medication and the effectiveness of treatment should be periodically monitored, especially in patients with osteoarthritis. Osteoarthritis in the acute stage: daily dose 7.5 mg (one tablet 7.5 mg or half a tablet 15 mg). If there is no effect, the dose can be increased to 15 mg per day (two 7.5 mg tablets or one 15 mg tablet). Rheumatoid arthritis, ankylosing spondylitis: daily dose 15 mg (two 7.5 mg tablets or one 15 mg tablet). (See the section “Use in certain categories of patients” below). Depending on the clinical effect, the dose may be reduced to 7.5 mg per day (one 7.5 mg tablet or half a 15 mg tablet). The daily dose of meloxicam 15 mg should not be exceeded. Use in certain categories of patients In elderly patients and patients with an increased risk of adverse reactions: in elderly patients, the recommended dose for long-term treatment of rheumatoid arthritis or ankylosing spondylitis is 7.5 mg per day. Patients at increased risk of adverse reactions should also begin treatment with meloxicam 7.5 mg per day. Use in patients with renal failure: In patients with severe renal failure on hemodialysis, the daily dose of meloxicam 7.5 mg should not be exceeded. In patients with mild to moderate renal impairment (creatinine clearance more than 25 ml/min), no dose reduction is required (see section "Precautions"). Use in patients with hepatic impairment: No dose reduction is required in patients with mild to moderate hepatic impairment (see section "Precautions"). Use in children: Melbek tablets are contraindicated in children and adolescents under 16 years of age.
Side effect
Clinical studies and epidemiological data suggest that the use of some NSAIDs (especially at high doses and during long-term treatment) may be associated with an increased risk of arterial thrombosis (eg, myocardial infarction or stroke). There have been reports of the development of heart failure, edema, and arterial hypertension associated with the use of NSAIDs. The most common adverse reactions are gastrointestinal disorders. Complications of a peptic ulcer may develop: perforation or gastrointestinal bleeding, sometimes fatal, especially in older people. Nausea, vomiting, diarrhea, flatulence, constipation, dyspepsia, abdominal pain, melena, vomiting with blood, ulcerative stomatitis, exacerbation of ulcerative colitis and Crohn's disease, gastritis have been reported. Adverse reactions are listed according to the frequency of occurrence according to the following scale: very often (? 1/10), often (? 1/100 to
Overdose
In case of acute overdose of NSAIDs, the following symptoms may be observed, which are usually reversible with maintenance therapy: weakness, drowsiness, nausea, vomiting and epigastric pain. Gastrointestinal bleeding may develop. Severe intoxication can lead to hypertension, acute renal failure, liver failure, respiratory depression, coma, seizures and cardiovascular failure. Just as when taking NSAIDs in therapeutic doses, overdose may cause anaphylactoid reactions. Treatment is symptomatic. The antidote is not known; in case of drug overdose, general supportive therapy should be carried out. Clinical studies have demonstrated that cholestyramine accelerates the elimination of meloxicam.
Interaction with other drugs
Other prostaglandin synthesis inhibitors, including glucocorticoids and salicylates (acetylsalicylic acid): simultaneous use of prostaglandin synthesis inhibitors increases the risk of ulceration in the gastrointestinal tract and gastrointestinal bleeding due to synergistic action. The combined use of meloxicam and NSAIDs is not recommended. Concomitant use with acetylsalicylic acid prescribed in anti-inflammatory doses (? 500 mg single dose or ? 3 g total daily dose) is not recommended. Oral anticoagulants, antiplatelet agents, systemic heparin, thrombolytic agents and selective serotonin reuptake inhibitors: increased risk of bleeding. The simultaneous use of NSAIDs and oral anticoagulants or heparin is not recommended for elderly patients. If it is impossible to avoid the simultaneous use of these drugs, careful monitoring of the effect of anticoagulants is necessary: careful monitoring of the INR (international normalized ratio) is required. Lithium: NSAIDs increase plasma lithium concentrations by decreasing renal excretion of lithium. Plasma lithium concentrations can reach toxic levels. The combined use of lithium and NSAIDs is not recommended. If such combination therapy is necessary, plasma lithium concentrations should be monitored at the beginning of treatment, when selecting the dose and when discontinuing meloxicam. Methotrexate: NSAIDs may decrease the tubular secretion of methotrexate and thus increase the plasma concentration of methotrexate. In this regard, concomitant use of NSAIDs is not recommended for patients receiving high doses of methotrexate (more than 15 mg per week). The risk of interaction with the simultaneous use of methotrexate and NSAIDs is also possible in patients receiving low doses of methotrexate, especially in patients with impaired renal function. If combination therapy is necessary, blood count and renal function should be monitored. Caution must be exercised if NSAIDs and methotrexate are used simultaneously for 3 days, because The plasma concentration of methotrexate may increase and, as a result, toxic effects may occur. Concomitant use of meloxicam did not affect the pharmacokinetics of methotrexate at a dose of 15 mg per week, however, it should be taken into account that the hematological toxicity of methotrexate is enhanced by concomitant use of NSAIDs. Contraception: Reduced effectiveness of intrauterine contraceptive devices with the use of NSAIDs has previously been reported, but this information requires further confirmation. Diuretics: The use of NSAIDs increases the risk of acute renal failure in patients with dehydration. Adequate hydration should be maintained in patients taking Melbec and diuretics. Before starting treatment, a kidney function test is necessary. Antihypertensives (eg, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, vasodilators, diuretics): NSAIDs reduce the effect of antihypertensives by inhibiting prostaglandins, which have vasodilating properties. The combined use of NSAIDs and angiotensin II receptor antagonists and ACE inhibitors enhances the effect of reducing glomerular filtration. In some patients with compromised renal function (eg, dehydrated patients or elderly patients with impaired renal function), concomitant use of an ACE inhibitor or angiotensin II antagonist and cyclooxygenase inhibitors may lead to a further deterioration of renal function, including the possibility of acute renal failure, such as usually reversible. This combination should be prescribed with caution, especially in elderly patients. Adequate hydration of the patient and monitoring of renal function after initiation of concomitant therapy and periodically during treatment are recommended. Cholestyramine, by binding meloxicam in the gastrointestinal tract, leads to its faster elimination. This interaction is of clinical significance. NSAIDs, by acting on renal prostaglandins, may enhance the nephrotoxicity of cyclosporine and tacrolimus. In case of combination therapy, renal function should be monitored, especially in elderly patients. With the simultaneous use of meloxicam and antacids, cimetidine, digoxin, no significant pharmacokinetic interactions were identified. The possibility of interaction with oral antidiabetic agents cannot be excluded. Risk of hyperkalemia: Certain drugs or therapeutic groups may contribute to the development of hyperkalemia: potassium salts, potassium-sparing diuretics, ACE inhibitors, angiotensin II receptor antagonists, non-steroidal anti-inflammatory drugs, heparins (low molecular weight or unfractionated), cyclosporine, tacrolimus and trimethoprim. The development of hyperkalemia may depend on the presence of risk factors. The risk of developing hyperkalemia increases with simultaneous use of the above drugs and meloxicam. Deferasirox: Concomitant use of meloxicam and deferasirox may increase the risk of gastrointestinal side effects. Therefore, these drugs should be taken simultaneously with caution. Pemetrexed: If meloxicam and pemetrexed are to be administered concomitantly in patients with mild to moderate renal impairment (creatinine clearance 45-79 mL/min), meloxicam should not be administered for at least 5 days prior to administration of pemetrexed, on the day of administration, and within 2 days after pemetrexed administration. If the combination of meloxicam and pemetrexed is necessary, close monitoring of the patient is recommended, especially due to myelosuppression and gastrointestinal side effects. In patients with severe renal impairment (creatinine clearance
Precautionary measures
Adverse reactions can be reduced by administering the lowest effective dose for the shortest possible time necessary to control symptoms. In case of insufficient therapeutic effect, the maximum recommended daily dose (15 mg) should not be exceeded. Concomitant use of other NSAIDs should be avoided as there may be an increased risk of toxicity without increasing treatment efficacy. The use of meloxicam in combination with other NSAIDs, including selective cyclooxygenase-2 inhibitors, is not recommended. Meloxicam is not recommended for patients who require relief of acute pain. If there is no improvement after several days of taking meloxicam, treatment should be reconsidered. Before starting treatment with meloxicam, it is necessary to clarify the medical history to determine whether the patient previously had esophagitis, gastritis, gastric or duodenal ulcers, and whether these conditions were completely cured. Due to the possible occurrence of relapse, patients with previous diseases should be under constant supervision while taking meloxicam. Gastrointestinal tract: As with other NSAIDs, potentially life-threatening gastrointestinal bleeding, ulceration or perforation may occur during treatment at any time with or without warning symptoms, regardless of the patient's history of serious gastrointestinal intestinal diseases. The above complications are usually more severe in older patients. The risk of gastrointestinal bleeding, ulceration or perforation is higher with increasing doses of NSAIDs, in patients with a history of ulcers or bleeding, and in elderly patients. These patients should begin treatment at the lowest effective dose. As with the use of other NSAIDs, special precautions should be taken when treating patients who have had or are having gastrointestinal diseases. Patients who experience gastrointestinal symptoms should be closely monitored. If ulcerative lesions of the gastrointestinal tract or gastrointestinal bleeding occur, Melbek should be discontinued. For elderly patients, as well as for patients receiving low doses of aspirin or other drugs that may increase the risk of gastrointestinal disorders, combination therapy (eg, misoprostol or proton pump inhibitors) should be considered. Patients with gastrointestinal toxicity, especially the elderly, should report the development of any unusual abdominal symptoms, especially early in treatment. Use with caution in patients receiving drugs that may increase the risk of ulceration or bleeding (elderly patients, patients receiving therapeutic doses of heparin, anticoagulants (eg, warfarin) or other NSAIDs, including acetylsalicylic acid prescribed in anti-inflammatory doses (at single dose or daily dose).Cardiovascular and cerebrovascular effects: Patients with hypertension and/or mild to moderate heart failure should be monitored during treatment with NSAIDs due to possible fluid retention and increased edema. Clinical blood pressure monitoring recommended in patients at risk of elevated blood pressure before and during treatment with meloxicam.Clinical studies and epidemiological data suggest that the use of some NSAIDs (especially in high doses and with long-term treatment) leads to a small increase in the risk of arterial thrombosis (for example, myocardial infarction or stroke, up to and including death). This risk cannot be excluded for meloxicam. Patients with cardiovascular disease or who have factors predisposing them to developing cardiovascular disease are at higher risk. In patients with uncontrolled hypertension, congestive heart failure, coronary artery disease, peripheral arterial disease and/or cerebrovascular disease, meloxicam should be prescribed only after assessing the benefit/risk ratio. The same analysis should be performed before starting long-term therapy in patients with risk factors for cardiovascular disease (for example, hypertension, hyperlipidemia, diabetes mellitus, smoking). Liver dysfunction: Episodic increases in serum transaminases or other liver function tests have been reported with the use of Melbec (as with most other NSAIDs). In most cases, this increase was small and transitory. If the identified changes are significant or do not decrease over time, Melbek should be discontinued and the identified laboratory changes should be monitored. Renal impairment: Episodic increases in serum creatinine or urea or other measures of liver function have been reported with the use of Melbec (as with most other NSAIDs). In most cases, this increase was small and transitory. If the identified changes are significant or do not decrease over time, Melbek should be discontinued and the identified laboratory changes should be monitored. In rare cases, NSAIDs may cause interstitial nephritis, glomerulonephritis, medullary renal necrosis, or nephrotic syndrome. In patients with end-stage renal failure on hemodialysis, the dose of Melbek should not exceed 7.5 mg. No dose reduction is required for patients with minimal or moderate renal impairment (that is, if creatinine clearance is more than 25 ml/min). NSAIDs inhibit the synthesis of prostaglandins in the kidneys, which are involved in maintaining renal perfusion. The use of NSAIDs in patients with reduced renal blood flow or reduced circulating blood volume may lead to decompensation of renal failure. After discontinuation of NSAIDs, renal function usually returns to baseline. Patients most at risk for this reaction are the elderly, those with dehydration, congestive heart failure, severe liver failure, cirrhosis, nephrotic syndrome, lupus nephropathy, or other severe illnesses. kidney; patients simultaneously taking diuretics, ACE inhibitors, angiotensin II receptor antagonists, as well as patients who have undergone major surgical interventions leading to hypovolemia. In such patients, diuresis and renal function should be carefully monitored when initiating therapy. The use of NSAIDs can lead to sodium, potassium and water retention and affect the natriuretic effect of diuretics. As a result, predisposed patients may experience increased signs of heart failure or hypertension. Clinical monitoring is recommended for these patients. Potassium levels should be monitored in patients with diabetes mellitus and in patients taking medications that may affect blood potassium levels. Weakened or malnourished patients may be less able to tolerate adverse reactions and such patients should be monitored carefully. As with other NSAIDs, caution should be exercised in the treatment of elderly patients who are more likely to have impaired renal, hepatic and cardiac function. Meloxicam, like other NSAIDs, can mask the symptoms of an infectious disease. The medicine contains lactose; patients with rare hereditary problems of galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption should not take Melbek tablets. Potentially life-threatening skin rashes (Stevens-Johnson syndrome and toxic epidermal necrolysis) have been reported with meloxicam use. Initially, a rash appears all over the body in the form of red round spots, often with a blister in the center. Additional signs: sores in the mouth, throat, nose, genitals, conjunctivitis (red, swollen eyes). Very often, life-threatening skin rashes are accompanied by flu-like symptoms. The rash can progress, often becoming confluent, and is accompanied by detachment of the epidermis. The highest risk of developing severe skin reactions is during the first weeks of treatment. If you have developed Stevens-Johnson syndrome or toxic epidermal necrolysis while using meloxicam. This medicine should never be restarted. If you develop a rash or other skin symptoms, stop using meloxicam and call your doctor immediately and tell your doctor what medications you are taking. The use of meloxicam may reduce fertility in women and is therefore not recommended for women planning pregnancy. If the ability to conceive in women is impaired or research is being conducted for infertility, it is necessary to consider discontinuing meloxicam. Concomitant use with pemetrexed Patients with mild to moderate renal impairment who are receiving pemetrexed should not take meloxicam for at least 5 days before pemetrexed administration, on the day of administration, and for 2 days after pemetrexed administration.
Storage conditions
Store at a temperature not exceeding 25°C in the original packaging.
Interactions
Melbek may reduce the effectiveness of antihypertensive drugs (beta-adrenergic receptor blockers, ACE inhibitors).
In women who use an intrauterine device, simultaneous use of Melbek may reduce its contraceptive effect.
You should not take meloxicam and other NSAIDs (especially acetylsalicylic acid and ibuprofen) at the same time, as this may increase the risk of ulcerogenic effects and gastrointestinal bleeding.
Meloxicam may enhance the effect of ticlopidine and heparin, which increases the risk of gastrointestinal bleeding.
Combined use with lithium salts is not recommended, given the decrease in lithium excretion by the kidneys under the influence of NSAIDs, which may cause the accumulation of lithium and the manifestation of its toxic effect.
Meloxicam should not be taken simultaneously with methotrexate, given the possibility of enhancing the toxic effect of the latter on the hematopoietic system. It is not recommended to use Melbek together with cyclosporine, since the risk of the nephrotoxic effect of the latter increases.
A pharmacokinetic interaction of meloxicam with other drugs at the metabolic stage is possible due to their effect on cytochrome P450 2C9 and/or cytochrome P450 3A4. With simultaneous use, no pharmacokinetic interaction of the drug with antacids, digoxin and furosemide was detected. Cholestyramine accelerates the elimination of meloxicam.
Interactions between the drug and oral hypoglycemic drugs cannot be excluded.
Interaction with other drugs:
The simultaneous use of two or more drugs from the NSAID group increases the ulcerogenic risk and the risk of gastrointestinal bleeding due to the synergistic effect of the drugs. It is not recommended to combine Melbek with lithium salts, since NSAIDs can reduce the excretion of lithium by the kidneys, which may cause its accumulation and contribute to its toxicity. When taken simultaneously, Melbek can increase the toxic effect of methotrexate on hematopoiesis, which requires constant monitoring of the dynamics of hemogram parameters. When taken simultaneously, Melbek enhances the effect of heparin and ticlopidine, resulting in an increased risk of gastrointestinal bleeding.
Melbek may reduce the contraceptive effect of the intrauterine device. When taking Melbek concomitantly with diuretics, patients should consume sufficient amounts of fluid. Meloxicam can reduce the effectiveness of antihypertensive drugs (ACE inhibitors, β-adrenergic receptor blockers). NSAIDs, ACE inhibitors and angiotensin II receptor antagonists have a synergistic effect on glomerular filtration, which can lead to the development of acute renal failure in patients with a history of impaired renal function. Meloxicam may bind to cholestyramine in the digestive tract, which will accelerate the elimination of meloxicam.
Melbek should not be combined with cyclosporine to avoid increasing the risk of nephrotoxicity of cyclosporine. The pharmacokinetic interaction of Melbek with digoxin, antacids and furosemide when taken simultaneously has not been established. It is possible that Melbek may interact with oral antidiabetic drugs.
Note!
Description of the drug Melbek table. 7.5 mg No. 30 on this page is a simplified author’s version of the apteka911 website, created on the basis of the instructions for use.
Before purchasing or using the drug, you should consult your doctor and read the manufacturer's original instructions (attached to each package of the drug). Information about the drug is provided for informational purposes only and should not be used as a guide to self-medication. Only a doctor can decide to prescribe the drug, as well as determine the dose and methods of its use.
Composition and release form:
Melbek tablets 7.5 mg.
The package contains 10 (5, 30) tablets. Melbek forte tablets 15 mg. There are 10 tablets in a package.
Melbek injection solution 1.5 ml. There are 10 ampoules in a package.
Melbek rectal suppositories 15 mg. There are 10 suppositories in a package.
1 tablet of Melbek contains: 7.5 mg (15 mg) of meloxicam. Excipients: Aerosil 200, povidone K30, lactose, magnesium stearate, crospovidone, sodium citrate, Avicel RN 102.
1 ampoule of Melbek contains: 15 mg of meloxicam. Excipients: glycofurol, meglumine, poloxamer 188, sodium chloride, glycine, water for injection, 1 m HCl or 1 m NaOH.