Summary
Negative trends in the demographic situation in the country, an increase in the number and severity of man-made accidents and disasters with significant medical and sanitary losses, and the complication of the domestic and foreign political situation due to the growth of terrorism, put forward additional requirements for improving the means and methods of organizing emergency medical care in the Russian Federation.
Among the priority areas of development, in addition to reforms in the organizational, financial and regulatory sectors, is the improvement of the material and technical base [11]. Until recently, as a means of stopping life-threatening external bleeding, emergency doctors only had a tourniquet and a pressure bandage [1,2]. But science has stepped far forward and now highly effective local hemostatic agents (HHAs) have become available for use, which can stop intense arterial and venous bleeding, are simple and easy to use, do not require special storage conditions, and have minimal undesirable effects [3,7, 9]. The list of such drugs, including domestic ones, is expanding from year to year. The wide-scale use of MGS as a first aid treatment for massive bleeding began with the serial release of the first model in the United States, the drug QuikClot [9]. Then this list progressively expanded. Specialists produced hemostatic agents from various materials, both organic and inorganic (HemCon, WuondStat, TraumaDex, Combat Gauze, Celox, etc.) [5]. The most effective of them began to be used in the armies of NATO countries and were included in life support kits [12]. In the Russian Federation, specialists from the Federal State Unitary Enterprise Research and Production Center "Pharmzashchita" FMBA of Russia created and, since 2010, launched into mass production the drug "GEMOSTOP" (RF Patent for invention No. 20091456-52 dated December 10, 2009), which is a representative of the class of synthetic zeolites and the first MGS on the domestic market [3]. In 2011, the drug was accepted for supply by the RF Armed Forces [3,6]. Accumulating experience in using this product in clinics and experimental laboratories, experts have come to the conclusion that the negative thermal effect of GEMOSTOP on tissue greatly limits its further use [5,7-10]. These prerequisites led to the creation of other MGS of a modified structure, registered under the trademark “GEMOSPAS” and “GEMOSPAS M”. Both drugs are intended to stop bleeding of moderate and high intensity.
The high requirements for equipping emergency medical equipment require a comprehensive assessment of their effectiveness and safety using biological models. Proven agents with high hemostatic efficiency - QuikClot, Celox and GEMOSTOP - should be used as reference drugs.
From the history of hemostatic agents
Medicinal herbs that have a hemostatic effect have been known for centuries.
Shepherd's purse, yarrow, and nettle leaves were used by ancient healers as hemostatic agents. The hemostatic effects of peppermint tincture, decoction of viburnum bark, and plantain leaves have long been known. Since time immemorial, dry moss has been applied to an open wound. But herbs hardly solved the problem, and mortality from blood loss remained high. More effective means were required, but things were not moving quickly. Only in 1883 did scientific research lead the French biologist G. Hayem to the discovery of the platelet. In 1890 , the role of calcium in blood clotting was proven. in 1916 and described in 1918 . In 1931 , a Canadian veterinarian accidentally discovered warfarin, an indirect anticoagulant. The main physiological anticoagulant, protein C, was discovered and described in 1976. Once the enemy is identified, it is easier to fight it. The sequential discovery and description of the action of three anticoagulants allowed scientists to begin the development of new hemostatic agents. Since the late 60s of the last century, the production of effective hemostatic drugs gradually began. Today medicine has a wide variety of modern hemostatic agents.
MATERIAL AND METHODS
The basis for the development of the experimental model was the work of R. Arnaud, N. Alam, A. Pusateri, V. Kheirabadi and their colleagues, who, to test the effectiveness of MGS, stopped bleeding from the femoral vessels in experimental animals [12-18]. The simplicity and clarity of the proposed model allowed it to be used by many specialists as the “gold standard” for testing hemostatic agents.
For the experiment, 16 Romanov breed rams with a body weight of 25.4-31.7 kg were selected. The experiment itself was carried out in a veterinary operating room in compliance with the requirements of the local ethics committee and the Declaration of Helsinki on the humane treatment of animals. At the preparatory stage, the experimental animals were deprived of food, but with free access to water. On the day of the experiment, before transportation to the operating room, a solution of tiletamine at a dose of 5 mg/kg was administered intramuscularly to induce anesthesia. Endotracheal anesthesia with isoflurane was chosen as a method of general anesthesia. For invasive blood pressure monitoring, a 5Fr sheath was placed in the carotid artery. Infusion of liquid media was carried out through an installed 6Fr catheter into the external jugular vein of the animal. In the groin area, directly under the inguinal fold, a 10-12 cm long incision was made with the intersection of the muscles and the vascular bundle, resulting in intense arteriovenous bleeding (Figure 1).
Rice. Fig. 1. Appearance of a wound in the groin area with intense arteriovenous bleeding.
After a latent period of 45 s, during which the animals lost from 18 to 25% of their blood volume, the bleeding was manually stopped using the studied MGS (Figure 2).
Rice. 2. Pouring MGS into the wound
Stop bleeding by temporarily applying pressure to the wound.
The use of funds was carried out in strict accordance with the current instructions. Simultaneously with the temporary stop of bleeding, the volume of lost bcc was replenished with a 5% solution of hydroxyethyl starch and a 0.9% solution of sodium chloride. After 7 minutes of manual compression, pressure on the wound was stopped and the result was assessed. When bleeding resumed, the hemostatic agent was reapplied according to the described scheme. Blood pressure was recorded for 60 minutes. The effectiveness of the MGS was assessed using the following indicators:
- the number of animals in which primary hemostasis was achieved;
- stability of hemostasis (HS), which was expressed through the formula:
SG = 1 - total number of recurrent bleeding (all animals) / total number of drug uses
- volume of blood loss;
- survival.
Local hemostatic agents being studied. The following MGS were assessed for comparative effectiveness:
- "GEMOSPAS" (MDK Medica LLC) is intended to stop external bleeding of varying intensity, including damage to large venous and arterial vessels, in a hospital setting to stop bleeding from parenchymal organs when traditional methods of hemostasis are ineffective. Can be used in first aid, medical care outside a medical organization, as well as in outpatient, inpatient settings and at home. It is a derivative of NaCaAX zeolite, which has high absorption capacity. The hemostatic effect is based on rapid moisture absorption. Upon contact with blood, a large volume of water is absorbed relative to the mass and volume of the drug, which leads to a local concentration of cellular and large protein components of the blood (including coagulation factors). This in turn induces the formation of a blood clot. In addition, the surface potential of zeolite promotes the activation of blood clotting factor XII and platelets. To enhance the hemostatic effect, the developers added calcium to the microcrystalline lattice. The latter is a cofactor in many parts of the coagulation cascade. According to the results of toxicological studies, the “Hemostatic dressing agent “GEMOSPAS” sterile” meets the requirements for medical devices that come into contact with the wound surface and blood;
- QuikClot (Z-Medica, Wellington Connecticut, USA). The basis of this preparation is zeolite, a natural mineral with a high ability to absorb moisture and a large surface area [13];
- Celox is a brand of chitosan-based hemostatic drugs manufactured by MedTrade Products Ltd. Available in various dosage forms, usually in the form of a powder or gel in a syringe tube. According to the manufacturer, it is capable of stopping bleeding of varying intensity, including damage to large arteries [15].
- GEMOSTOP (FSUE SPC "Pharmzashchita" FMBA of Russia) is intended to stop intense external bleeding, incl. with damage to large arteries. It is a material based on a mineral sorbent - granular zeolite.
Main contraindications and side effects
Hemostatic sponges should not be used to stop arterial bleeding, in case of damage to large vessels, as well as in case of individual intolerance to components and drugs of the nitrofuran series, purulent wounds and pyoderma.
Side effects may occur if used incorrectly or inappropriately, especially in contaminated alveolar spaces. With rare exceptions, allergies may occur, in which case it is necessary to stop using the drug.
Results and discussion
During the experiment, the procedure for using hemostatic agents, in general, remained standard - pouring the drug as close as possible to the source of bleeding, placing a cotton-gauze pad over the wound and manual compression for 7 minutes. If a relapse occurred, MGS was re-filled in the amount necessary to fill the entire wound cavity (taking into account the washout of part of the drug by a continuous flow of blood) and the algorithm of actions was repeated. After the wound was inflicted, a stream of profuse bleeding occurred. Within 45 seconds, blood loss was 347.14±117.26 ml. Average blood pressure decreased from 80-90 to 20-23 mm Hg. By 4-6 minutes there was a slight increase in SBP associated with a temporary stop of bleeding, compensatory mechanisms and hemodilution. After the start of infusion therapy, the average blood pressure of animals in which hemostasis was achieved stabilized at values of at least 60 mmHg.
In the group where GEMOSPAS was used, primary hemostasis was achieved in 2 animals (50%). In individuals 3 and 4, relapse of bleeding was observed twice. After repeated additions of the product, final hemostasis was achieved. Thus, the total number of recurrent bleeding was 4, per 8 uses of the drug in all animals. The integral indicator reflecting the stability of hemostasis is 0.5. All animals survived the experiment. The average total blood loss was 1775.57±202.81 ml.
In the second experimental group, where Celox was used, primary hemostasis was achieved in only 1 individual (25%). In animals 2, 3 and 4, repeated resumption of bleeding was noted in the form of intense leakage of blood from under the cotton-gauze pad. In only one animal, hemostasis occurred immediately after repeated use of MGS; in the remaining two animals, relapse occurred again, which required 2-3 additions of a hemostatic agent. The total number of recurrent bleeding was 6, per 10 uses of the drug in all animals. The stability of hemostasis is 0.4. One animal died within 30 minutes of the experiment.
from an irreversible decrease in blood pressure. The average total blood loss was 1668.0±102.03 ml.
In the third experimental group, QuikClot was used. In fact, all recorded parameters in the other groups were compared with it, since the effectiveness of this remedy has already been repeatedly proven in numerous studies and experiments on animals. In this group of animals (n=4), primary hemostasis was achieved in 1 case (25%). In 2 and 3 individuals, resumption of bleeding was noted 1 and 2 times, respectively. The total number of recurrent bleeding was 3, per 7 uses of the drug. The stability of hemostasis is 0.6. All animals survived. The average total blood loss was 1536.67±117.86 ml.
In the fourth experimental group, the drug GEMOSTOP was used. When used on four experimental animals, primary hemostasis was achieved in 2 cases (50%). In 3 and 4 individuals, resumption of bleeding was noted once and twice in a row, respectively. The total number of recurrent bleeding was 3, per 7 uses of the drug. The stability of hemostasis is 0.6. All animals survived. The average total blood loss was 1632.27±121.85 ml. Summary data on the experiment and analysis results are presented in the table.
The objective of our study was to compare the effectiveness of two domestic (GEMOSPAS, GEMOSTOP) and two foreign (QuikClot, Celox) agents for local hemostasis in the wound during intense arteriovenous bleeding. As you can already see, no statistically significant differences were obtained for all control points. It is impossible to say with certainty that any of the presented means showed the best result.
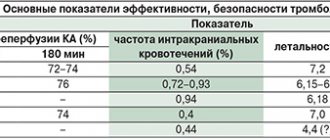
Summary data on the effectiveness of the studied MGS
| Performance parameters | GEMOSPAS | Celox | QuikClot | GEMOSTOP | R |
| Primary hemostasis | 2 (50%) | 1 (25%) | 1 (33.3%) | 2 (67%) | 0.79 |
| Total number of bleeding recurrences | 4 | 6 | 3 | 3 | 0.05 |
| Total number of drug uses | 8 | 10 | 7 | 7 | 0.03 |
| Stability of hemostasis (cu) | 0,5 | 0,4 | 0,6 | 0,6 | 0.87 |
| Average total blood loss (ml) | 1775,57±202,81 | 1668,0±102,03 | 1536,67±117,86 | 1632,27±121,85 | 0.76 |
| Survival | 100 | 75 | 100 | 100 | 0.08 |
The integral indicator “stability of hemostasis” that we developed well reflects the situation with the number of relapses and repeated uses of MGS. Despite the apparent differences between the groups at first glance, they were not confirmed. A significant drawback in the experiment is the small number of animals caused by shortcomings in logistics. It is advisable to use at least 6-8 for each group, then the statistical data will be more reliable. With strict selection of animals, it is necessary to conduct a screening study of the hemostasis system in the preparatory period, because significant differences in performance may affect the results. Some researchers note that the model we applied is not completely adequate. It is believed that with complete intersection of the vessels, hemostasis quickly occurs due to the protective contraction of the muscular lining of the artery and the screwing in of the vessel lining. According to L. Littlejohn et al., with complete intersection of the vessels, wound tamponade and/or a reliable pressure bandage are not inferior in effectiveness to MGS [19]. In the alternative model of marginal injury to the femoral artery proposed by scientists, even the modern standard NATO hemostatic agent “CombatGauze” ensures survival of only 33% of animals, and primary hemostasis has never been noted at all. The authors indicate that an ideal experimental model for future studies should achieve hemostasis of only 30-50% using CombatGauze as the standard [17]. Making the experimental model heavier will make it possible to more clearly analyze the real usefulness of the tested MGS, especially at the stages of testing and implementation. More careful selection of animals and their randomization into groups is necessary. We also consider it inappropriate to use additional wound packing and the application of tight pressure bandages over the wounds in the experiment, since this can seriously distort the results of the experiment. Moreover, it is difficult to achieve standardization of dressings from case to case.
Despite the above disadvantages and limitations in this experiment, preliminary conclusions can be drawn.
Bleeding during or after surgery is one of the most significant complications in surgical practice. In modern surgery, the number of surgical interventions on organs, which may be accompanied by severe bleeding, is increasing (surgeries on the heart, blood vessels, parenchymal organs, liver, pancreas, kidneys). The use of traditional methods of hemostasis (mechanical, thermal hemostasis) can not only be ineffective, but also lead to the development of serious complications.
The increasing number of patients with comorbidities and frequent use of anticoagulants is an important factor that contributes to the high risk of surgical bleeding. Diffuse bleeding from large wound surfaces in patients with coagulopathy is particularly difficult for hemostasis.
It has been established that almost 30% of operations are accompanied by bleeding, while in cardiovascular surgery this figure approaches 50% [1]. Excessive bleeding complicates surgery and often leads to negative consequences, including longer hospital stays, increased healthcare volumes, and increased treatment costs.
Massive bleeding often requires a blood transfusion if hemostasis cannot be achieved.
These procedures often carry significant risks, including bacterial infections [2], delayed recovery [3], and death.
The length of hospital stay can increase by 1.5–2.5 times in patients with hemostasis/transfusion disorders [1]. These complications are the cause of higher medical costs [4].
Intraoperative and postoperative bleeding are potential risks associated with all surgical procedures, and the consequences of prolonged bleeding are costly and often seriously detrimental to the health of patients. Therefore, control of bleeding is a key component of successful surgery, and the most important requirement in all surgical procedures is to achieve and maintain hemostasis during and after surgery.
A recent retrospective analysis of more than 1.6 million procedures performed in the United States (Stokes et al., 2011) found an average bleeding complication rate of 29.9% [1]. In Fig. 1 shown
Rice. 1. Frequency of complications due to bleeding in different areas of surgery. proportion of patients with hemorrhagic complications depending on the type of surgery. In all areas of surgery (cardiac surgery, vascular surgery, thoracic surgery, solid organ surgery, general surgery, knee/hip replacement, reproductive organ surgery, and spine surgery), complications associated with bleeding inevitably increased the patient's ICU stay and costs. for inpatient treatment.
If the surgeon provides timely hemostasis, this reduces the duration of the operation, wound exposure, reduces the need for blood transfusion, optimizes therapy in patients receiving anticoagulant drugs, and has a positive effect on the healing of the surgical wound and postoperative rehabilitation [5].
Hemostasis during surgery is achieved:
- by mechanical means (surgical intervention taking into account the anatomical features of the operation area, ligation and clipping of blood vessels, tamponade);
— the use of coagulation devices operating on various physical principles (electrocoagulation, photocoagulation, laser, ultrasound, radio frequency, microwave, argon plasma coagulation, etc.);
— chemical methods (application hemostatic agents affecting the blood coagulation system).
There are possibilities of influencing the blood coagulation system through transfusion of blood components, coagulation factors, and fibrinolysis inhibitors. However, these drugs have a systemic effect and do not focus their action on the area of bleeding. In contrast, local hemostatic agents act in a targeted manner and can be used in cases of bleeding (wound surface of a parenchymal organ, cancellous bone, etc.), when physical and systemic methods of hemostasis may be ineffective. They are also especially valuable when there are difficulties in surgical access to the source of bleeding, which involves the use of topical hemostatic agents that can “flow” to the site of bleeding, stop it and seal the defect.
At the same time, there is currently an increasing need for new hemostatic agents that can improve clinical results in surgery and minimize the economic burden on medical centers.
This has stimulated the ongoing development of new drugs, including oxidized regenerated cellulose (ORC), porcine gelatin, bovine collagen, polysaccharide powders, and thrombin-based agents [6].
Recently, a wide range of surgical hemostatic agents have been developed for use in vascular surgery [7]. These agents vary significantly in their mechanism of action, composition, application, adhesion to wet or dry tissues, immunogenicity and cost. Broadly speaking, these substances can be divided into three categories: hemostatic agents, sealants, and adhesives.
Hemostatics can be divided into mechanical, active and fluid. Sealants are divided into fibrin and synthetic. Adhesives used in surgery are classified according to their composition into cyanoacrylate and albumin + glutaraldehyde (Table 1).
Table 1. Local hemostatic agents used in vascular surgery
In particular, the category of flowable hemostatic agents can be divided into two additional classes: porcine gelatin preparations that can be combined with any of three types of thrombin (bovine, human plasma thrombin, or recombinant), and bovine collagen materials with the addition of human plasma thrombin. In the literature, flowable hemostatic agents are defined as flowable sealants based on a gelatin-thrombin matrix.
Let's take a closer look at the Floseal matrix, which is a flowable hemostatic matrix used as an additional tool in achieving hemostasis in situations where attempts to stop bleeding by vessel ligation and/or other traditional methods are unsuccessful or practically impossible. The drug can be used for any type of bleeding and in all areas of surgery and types of surgical interventions, with the exception of ophthalmic operations. The two key components of Floseal, the gelatin matrix and thrombin, work in synergy to accelerate the formation of a stabilized clot [8]. Floseal is a hemostatic agent that can quickly and effectively stop bleeding ranging from capillary parenchymal to profuse pulsatile arterial [9, 10].
Quantitative indicators of hematopoiesis when using the Floseal matrix:
— stops bleeding within 2 minutes (or faster, the median is indicated) [11];
— in 97% of cases, stops any bleeding in 6-10 minutes [11];
— the mechanism of action is realized both at the beginning and at the end of the coagulation cascade [9];
— effective hemostasis is also achieved in patients with coagulopathies [10].
The combination of gelatin granules and thrombin in the Floseal hemostatic matrix promotes the formation of a mechanically stable clot over the entire surface of the wound. Subsequently, the clot completely resolves as the wound heals physiologically within 6-8 weeks.
Today it is a hemostatic agent that has proven clinical effectiveness across the entire spectrum of bleeding intensity, ranging from diffuse wound bleeding to pulsating arterial bleeding.
Application of Floseal (flowable hemostatic matrix) in surgery
In cardiovascular [12–16] and thoracic surgery [17], Floseal provides successful and faster hemostasis in cardiac and vascular interventions compared with other agents, as well as in the case of extensive pulmonary resection, compared with standard procedures. In addition, the use of Floseal reduces the number of postoperative complications. G. Nasso et al. [46] conducted a retrospective study of the effectiveness of Floseal in cardiovascular surgery, in which it was compared with other hemostatic agents. Floseal demonstrated a significantly higher rate of successful hemostasis. Effective hemostasis contributed to a reduction in the number of patients who required blood transfusions, and when blood transfusions were required, they were performed in significantly smaller volumes [47].
In spinal surgery [18-20] and neurosurgery [21-24], the use of Floseal provides faster hemostasis, reduced blood loss, a less pronounced drop in hematocrit, reduces the need for transfusion of blood components and shortens the operating time for spinal and neurosurgical interventions compared to other drugs (Surgiflo, Gelfoam+thrombin) or mechanical methods. Studies in the field of neurosurgery have shown that the use of Floseal led to rapid achievement of hemostasis in 94-100% of cases during neurosurgical intervention [48].
General surgery - the use of Floseal reduces bleeding time and blood loss, which increases the likelihood of the patient being discharged from the hospital on the day of surgery, for example after hemorrhoidopexy [25], ensures successful hemostasis and reduces blood loss, and, accordingly, the need for blood transfusions during liver resection [26], reduces the number of conversions during laparoscopic interventions [27] and is an indispensable method for the treatment of traumatic injuries of the spleen, providing hemostasis in emergency surgery [28], reducing operation time and the duration of drainage of the surgical wound, as well as the number of bed days [29].
In gynecology [30—37], the use of Floseal reduces intra- and postoperative blood loss, the need for blood transfusions, shortens the period of hospitalization after myomectomy, excision of endometrioma, stops postpartum hemorrhage from the placental site, and provides additional advantages in terms of preserving the ovarian reserve during the removal of ovarian cysts.
In urology [38–44], the use of Floseal during radical prostatectomy, partial nephrectomy and other urological operations leads to a decrease in blood loss and/or the need for blood transfusions, and reduces the number of postoperative complications and length of hospitalization compared to traditional hemostasis techniques. The use of Floseal allows you to avoid clipping the renal artery in order to reduce the intensity of bleeding, reducing the risk of developing renal failure due to temporary interruption of blood supply to the kidney[45].
Conducted studies [49] have demonstrated similar results in other areas of surgery, such as orthopedic surgery, liver surgery, kidney surgery, gynecological surgery and thyroidectomy, and nosebleeds.
In addition to the time of hemostasis, a comprehensive assessment of the effectiveness of a local hemostat should include other indicators, such as changes in length of hospitalization, the number of re-interventions for bleeding, length of stay in the operating room, the effect on the number of intra- and postoperative complications, and cost-effectiveness.
Duration of hospitalization
The use of Floseal can reduce hospitalization time, avoid complications, transfusions and reduce treatment costs compared to Surgiflo in cardiac surgery [50], general surgery, spinal [51] and neurosurgical patients.
The use of Floseal in urology reduced pain and discomfort in patients, allowed for earlier removal of transurethral catheters, shortened the duration of hospitalization after percutaneous nephrolithotomy, and reduced the need to use a nephrostomy catheter [52].
In general surgery, Floseal reduced blood loss and/or the need for blood transfusions, allowing the patient to be discharged from the hospital on the day of hemorrhoidopexy [53]. Also, the use of Floseal reduced the conversion rate of laparoscopic operations for acute cholecystitis [54].
Repeated interventions for bleeding
The use of Floseal reduced the need for surgical exploration for bleeding [55].
In studies [56–60] analyzing clinical outcomes in cardiac surgery, the use of Floseal was associated with a reduction in the rate of re-intervention for bleeding compared with Surgiflo and other hemostatic agents (Surgicel Nu-Knit, Gelfoam, oxidized regenerated cellulose).
Another similar study [61] also demonstrated a reduction in the rate of re-intervention for bleeding in spinal surgery.
Time spent in the operating room
The use of Floseal [62-68] reduces the duration of cardiovascular, spinal, neuro- and general surgical interventions.
According to the Premier database (USA), the use of Floseal [69] reduces the time a cardiac surgery patient spends in the operating room to a greater extent than Surgiflo.
Number of intra- and postoperative complications
Floseal reduces complications, mainly in cardiovascular, thoracic, general surgery and urology. Floseal has demonstrated a reduction in complications compared with Surgiflo [70].
In urology, Floseal reduced the cost of each radical prostatectomy by $315 due to a reduced incidence of lymphocele [71]. The use of Floseal can reduce intra- and postoperative costs in cardiovascular, spinal, gynecological, urological, general surgery and neurosurgery. In cardiovascular and thoracic surgery, Floseal demonstrates successful achievement of hemostasis in a short time, reduces the incidence of de novo
and rebleeding, which reduces the need for blood transfusions, intra- and postoperative costs compared to other hemostatic agents, including Surgiflo [72-76].
Cost of treatment
The use of Floseal during surgery leads to lower costs throughout the entire period of inpatient treatment.
Floseal reduces hospital costs, as well as the cost of treatment overall, compared to other agents in cardiac, neurosurgical and spinal patients.
Reduced resource consumption provides savings in treatment costs.
The use of Floseal often reduces the need for blood transfusions, potentially saving $522 to $1183 per unit volume of blood product in hospitals in the United States and Europe [77]. Studies have shown that the use of other hemostatic agents during surgery increased treatment costs ranging from 14.5 to 69% compared with the use of Floseal [78, 79].
Comparative clinical studies of floseal and Surgiflo
The following studies present a comparative analysis, which shows that the use of Floseal reduces the need for blood transfusions, shortens the operating time compared to Surgiflo, and most importantly, these advantages are achieved by using a smaller amount of the drug [80, 81].
In the first economic study (Ramirez et al., 2018), the use of Floseal alone was shown to reduce costs (operative time, length of stay, blood transfusions, less hemostatic required) compared with the use of Floseal + gelatin/thrombin sponge in of $317,959 in a hospital with an average volume of patients (130 spinal procedures per year). It should be noted that the size of the savings varies depending on the annual number of interventions.
In the case of a high patient flow and a significant number of operations, for example, 300 spinal surgeries per year, the amount of savings will approach ¾ million dollars ($733,752) per year, and with 50 spinal surgeries per year, the annual savings will be $122,292. Analysis of the results allows us to conclude that cost savings when using Floseal compared to Floseal + gelatin/thrombin sponge in spinal surgery occur across the full range of clinical outcomes and resource costs.
The presented figures are consistent with the results of other similar studies analyzing the pharmacoeconomic indicators of the use of hemostatics (see Table 3).
Table 3. Results of hemostatic use in both groups Note. SS—adjusted mean; CI—confidence interval; N/A – not applicable; *—odds ratios from logistic regressions for all binary outcome variables.
Table 2. Comparison of economic indicators when using Floseal
The second study (Makhija et al., 2017) compared Floseal and Surgiflo with thrombin in spinal surgery. Savings were shown to be at least $61,251 per year ($151 per procedure and $547 per major procedure) for the average hospital (182 radical procedures and 59 major spinal procedures per year). These savings are achieved by reducing the need for blood transfusions and reducing operating room time by 27 hours.
A third study (Tackett et al., 2014) compared Floseal with non-flowing local hemostatic packs or sponges (Surgicel Nu-Knit and Gelfoam) in cardiovascular surgery. The results indicate annual savings of up to $5.38 million ($8,960 per case) when using Floseal during 600 different cardiac surgeries (including coronary, valvular, combined coronary and valvular, and aortic surgeries) , when it was the prevented complications (33 major and 76 minor complications) that contributed to the reduction in the cost of treatment.
In a fourth study (Makhija et al., 2017) comparing the use of Floseal and Surgiflo in cardiac surgery, the authors concluded that the former avoided 11 major complications, 31 minor complications, 9 surgical revisions, 79 blood product transfusions and a cumulative savings of 260.3 h in the operating room compared to Surgiflo. In total, annual savings of $1.5 million were stated (i.e., $6256 per intervention) for an average specialized hospital performing 245 cardiac surgeries per year.
It is known that the use of local hemostatic agents based on a gelatin matrix and thrombin in addition to traditional methods helps to effectively stop bleeding during cardiac surgery. A prospective randomized trial (Nasso et al., 2009) compared Floseal with traditional local haemostatic agents in a mixed cohort of elective cardiac and thoracic aortic surgery [86]. The Floseal group included 209 patients, the group of alternative local hemostatics (comparison group) - 206 patients. Hemostatic agents in the comparison group included hemostatic swabs or sponges consisting of oxidized regenerated cellulose or purified porcine skin gelatin. The study used the following endpoints: rate of intraoperative hemostasis, time to achieve hemostasis, total number of postoperative bleeding, rate of blood transfusions, rate of re-interventions for bleeding, rate of postoperative complications, length of stay in the intensive care unit.
In the Floseal group, a significantly higher rate of successful hemostasis was noted, achieved in a shorter period of time ( p
<0.001).
The incidence of postoperative bleeding and blood component transfusions was significantly lower in the Floseal group ( p
< 0.001). There was no statistically significant difference in the rate of revisions for bleeding and the rate of minor complications, but there were significantly fewer of them in the Floseal group among patients with obvious intraoperative bleeding. The benefits of Floseal also extended to patients undergoing surgery under systemic hypothermia. The authors concluded that the use of local hemostatic Floseal in addition to traditional surgical methods of stopping bleeding made it possible to effectively stop episodes of intraoperative bleeding. Judicious use of this hemostatic agent reduces the need for transfusions of blood components and the frequency of re-interventions for bleeding.
Since the early 2000s, a significant number of prospective randomized clinical trials have shown the advantages of flowable hemostatic agents (Floseal Matrix hemostatic matrix) compared to non-flowable ones [87–89]. S. Tackett et al. analyzed the results of the clinical use of flowable hemostatic matrices in cardiac surgery. The retrospective analysis included cardiac surgery patients from 2006 to 2012. Data for analysis were taken from the Premier prospective studies database (USA), which contains information on approximately 25% of all patients who received inpatient care. The database was created to establish benchmarks for quality of care and resource use. The analysis included coronary artery bypass grafting (CABG), aortic surgery, valvular surgery, or combined valve surgery with CABG using Floseal or Surgiflo.
Depending on the hemostatic matrix used, 3 groups were formed: (A) Floseal or surgiflo; (B) Floseal or Surgiflo with the use of additional sealants (fibrin, powder); © Floseal or Surgiflo with concomitant use of non-flowing hemostatic agents with or without thrombin. The authors assessed the incidence of complications, blood transfusions, re-interventions for bleeding, mortality, length of hospitalization and surgical time.
Group A included 4480 cases of Floseal and 326 cases of Surgiflo (Table 3). The results indicate that Surgiflo patients were associated with an increased risk of multiple adverse outcomes, including major and minor complications, surgical revisions, blood transfusions, and longer surgical times overall, compared with the Floseal group. There were no significant differences between the groups in terms of mortality and duration of hospital treatment.
The authors showed the advantage of Floseal over Surgiflo due to a significant reduction in the incidence of any complications in the group of surgical patients. This study is the first and largest known analysis of the effectiveness of two hemostatic matrices in a large sample of surgical patients (of 314,415 cardiac surgeries, 4,806 cases in group A, 8,376 in group B, and 10,916 cases in group C met the inclusion criteria). Access to the Premier hospital database (USA) allowed the authors to analyze the outcomes of interventions, including complications and cases of blood transfusions in cardiac surgery practice.
S. Tackett et al. [90] examined the role of improved clinical outcomes in saving hospital resources. While the effectiveness of local hemostatic agents in cardiac surgery has been widely reported, assessment of their economic benefits is still limited. This study quantifies the reduction in hospital costs and improved clinical outcomes resulting from the use of a flowable matrix compared with non-flowable local hemostatic agents in cardiac surgery.
Using clinical outcomes from prospective RCTs as a basis, the authors used a cost-effect approach to model the economic consequences of different drugs. Clinical outcomes following the use of a flowable hemostatic matrix (Floseal, Baxter Healthcare Corporation) were compared with those using a non-flowable topical hemostatic agent (Surgiflo Nu-Knit, Ethicon-Johnson & Johnson). In the comparison group, Surgiflo Nu-Knit was used in 60.2% of cases, GELFOAM - in 39.8%. The authors analyzed the costs of treating complications, blood transfusions, re-interventions, and time spent in the operating room. The cost of cardiac surgery was estimated at $2012, based on published clinical practice data in the United States. Variability in incremental costs (or savings) for hemostatic agents in the comparison group was assessed on an annualized basis. Key outcome clinical data were collected from prospective RCTs or directly from clinical investigators [91]. The results of using hemostatic agents are presented in table. 4.
Table 4. Study endpoints, outcomes and complications in patients with intraoperative bleeding [92] Note. * — the time to achieve hemostasis included the time from the moment of decannulation to the moment of suturing the sternum, NS — the differences are not significant.
The results indicate that the use of only a flowable hemostatic matrix during 600 different cardiac surgeries per year leads to a reduction in the number of major complications by 33 episodes, minor complications by 76, repeated interventions for bleeding by 54, blood transfusions by 194, and also reduces the time 242 hours in the operating room. These improvements in clinical outcomes are equivalent to annual cost savings (savings) of $538 million, primarily due to avoided complications.
An assessment of cost dynamics in a cost-effect model based on 600 cardiac surgeries shows a reduction in the incidence of complications (major complications, minor complications, re-interventions for bleeding, blood transfusions and operating room time) and economic costs (Table 5).
Table 5. Clinical and economic impact per year for one cardiac surgery center performing 600 procedures per year Note. * - the difference in the incidence of serious complications between the FLOSEAL group and the comparison group was statistically insignificant. However, the reduction in serious complications was not statistically significant.
The results of the analysis confirm the position that the routine use of flowable matrix hemostatic agents in cardiac surgery instead of non-flowable ones allows for a significant reduction in the cost of treatment.
A retrospective comparative study by D. Makhija et al. [93] found that the use of the Floseal hemostatic matrix in cardiac surgery was accompanied by a significant reduction in the risk of complications, the frequency of blood transfusions, re-interventions and the duration of surgery compared with the SURGIFLO matrix. These advantages naturally lead to resource savings in cardiac surgery practice.
The authors assessed the cost/effect ratio of the use of two flowable hemostatic matrices (Floseal or Surgiflo) in cardiac surgery in American clinics.
The analysis included the duration of surgery, the incidence of major and minor complications, reoperations, and blood component transfusions. The cost of the above procedures and interventions was taken from the Healthcare Cost and Utilization Project's National Inpatient Sample (NIS) database from 2012, which was $2015. To calculate savings, the model was based on a clinic performing 245 cardiac surgeries per year, which is the average for clinics in the NIS database. To assess the reliability of the model, univariate sensitivity analysis and probabilistic sensitivity analysis were performed.
The results indicate that Floseal avoided 11 major complications, 31 minor complications, 9 surgical revisions, 79 blood transfusions, and reduced operating room time by 260.3 hours. These improvements in clinical outcomes correspond to an annual net cost savings of $1,532,896. In Fig. 2 shown
Rice. 2. Projected cost change for using floseal in an average US clinic. that despite Floseal's higher purchase prices ($48,519 for 245 cardiac surgeries), the expected annual cost savings with Floseal are much higher ($1,532,896 for 245 cardiac surgeries) compared to Surgiflo.
Projections for savings remain stable even taking into account the diversity of clinical situations according to univariate (1.3-1.8 million) and probabilistic sensitivity analyzes (911 thousand - 2.4 million) (Fig. 3).
Rice. 3. Cost Savings with Floseal. 1
Thus, published data from comparative studies of the effectiveness of Floseal and Surgiflo demonstrate significant savings in treatment costs in US clinics.
Results from a retrospective comparative analysis of two active flowable hemostatic matrices (Floseal and Surgiflo with thrombin) demonstrated significantly increased resource costs and complication rates (operative time, transfusion requirements, and amount of matrix used) with Surgiflo compared with Floseal in radical surgery. spine. Even longer operations were observed in the case of extensive spinal interventions.
The study methodology included a sample of inpatients for 2012 (NIS-National Inpatient Sample). Costs of hemostatic matrices, blood component transfusions, and operating room time were taken from previously published data. Additionally, univariate and probabilistic sensitivity analyzes were performed.
The results of the study indicate that in a hospital with an average annual surgical load, the use of Floseal instead of Surgiflo saved the transfusion of 3 units of blood derivatives per patient and 27 hours of work in the operating room. Accordingly, savings of up to $151 were noted for each radical procedure and $574 for each major spinal procedure (Table 6).
Table 6. Summary of Resource Savings Analysis of low/high surgical activity hospitals confirms similar cost savings data. A probabilistic sensitivity analysis showed that Floseal provided cost savings in 76% of radical spine simulations and 97% of major spine simulations.
Thus, the economic analysis demonstrates that the use of the Floseal hemostatic matrix instead of Surgiflo to induce hemostasis in radical and major spinal interventions leads to significant cost savings in US clinics.
A retrospective analysis of the Premier database (P. Faivre et al., 2015) showed that the use of the Floseal hemostatic matrix during radical spinal surgery (spinal fusion/fusion of 2-3 vertebrae) reduces the time spent in the operating room by 8.84 minutes ( p
<0.0001), the frequency of blood transfusions by 0.2% (
p
<0.0001) and reduces the volume of hemostatic used by 3.35 ml (
p
<0.001) compared to Surgiflo/Trombin [92].
In case of extensive interventions (spinal fusion/respondylodesis of 4 vertebrae or more), the use of Floseal reduces the operation time by 26.94 minutes ( p
<0.001), the volume of hemostatic used by 1.52 ml (
p
<0.008) compared with Surgiflo/Trombin.
For comparison, a cost-effect analysis was conducted for the use of Floseal and Surgiflo/Trombin in spinal surgery in 4 European countries.
A cost-effect model was constructed using data from a retrospective study and included operative time, blood transfusion requirements, and volume of hemostatic agent used.
The cost of blood components was taken from official sources. The calculation was made for 1000 radical and 1000 extensive interventions per year.
Using Floseal instead of Surgiflo/Trombin resulted in savings of €56,000 (Germany) to €344,000 (Netherlands) per 1000 radical procedures and €179,000 (Germany) to €540,000 (UK) per 1000 major procedures. Savings amounted to more than €100 for each radical intervention and more than €200 for each extensive intervention.
This analysis demonstrates that the use of Floseal instead of Surgiflo/Thrombin as an adjunctive hemostatic agent in radical and major spinal procedures results in significant savings in hospital costs, and the specific amounts of savings vary between countries.
In conclusion, uncontrolled bleeding can have significant clinical and economic consequences, including increased medical costs and poorer patient outcomes.
A comprehensive discussion of the agents used to achieve local hemostasis will facilitate their more optimal use in the operating room. Proper and correct use of these drugs will increase the efficiency of the surgical team, improve patient outcomes, and optimize the costs of medical institutions.
Currently, active liquid agents are considered the most effective of all hemostatic agents. In this regard, the review provides a detailed comparative analysis of the effectiveness of the flowable active hemostatic matrix Floseal and other hemostatic agents widely used in global surgical practice.
Floseal shows significantly higher effectiveness in terms of time to hemostasis, reduction in hospital stay, re-intervention rate for bleeding, length of stay in the operating room, number of intra- and postoperative complications and increased cost-effectiveness.
The author declares no conflict of interest.
The author declare no conflicts of interest.
Information about authors
Zemlyanoy A.B. — +79265932097
Corresponding author:
Zemlyanoy A.B. — email
Varying each model parameter one at a time in the one-way sensitivity analyzes showed that FLOSEAL's savings for the same 245 patient cohort ranged from 1,317,675 USD— 1,819,536 USD. The most important cost drivers were: (1) the ratio of cardiac surgery cases requiring surgery revision for bleeding, (2) the ratio of minor complications, (3) the ratio of major complications, (4) the cost of the base cardiac surgery , (5) the percentage of major complications, (6) the percentage of cardiac surgery cases requiring surgical revision for bleeding, (7) the percentage of minor complications, (8) the surgery time ratio, (9) the cost of cardiac surgery with blood product transfusion, and (10) the cost of operating room time.
CONCLUSIONS
- The drug "GEMOSPAS" is not inferior to Russian and foreign analogues in terms of selected efficiency parameters;
- When analyzing the results, it is necessary to adhere to the “stability of hemostasis” indicator, which helps prevent false conclusions;
- For full statistical processing of the results, one should adhere to a clearly planned experimental methodology on a sufficient amount of material;
- Based on the results of preclinical medical-biological and toxicological studies, the biological safety of the medical device has been confirmed, “GEMOSPAS” can be recommended for clinical trials.
BIBLIOGRAPHY
- Bagnenko S. F., Miroshnichenko A. G., Vertkin A. L., Khubutia M. Sh. Guide to emergency medical care. - M.: GEOTAR-Media, 2007. - 816 p.
- Belevitin, A. B., Samokhvalov I. M., Fomin N. F. et al. The problem of temporarily stopping external bleeding in injuries of the great vessels of the extremities from N.I. Pirogov to the present day // Bulletin of the Russian Military Medical Academy. - 2010. - T. special issue. — P. 13-18.
- Boyarintsev V.V., Samoilov A.S., Nazarov V.B. et al. History and current state of the problem of stopping external massive bleeding in Russia and abroad // Moscow Surgical Journal. — 2011.—№4. -WITH. 51-57.
- Boyarintsev V.V., Yudin A.B., Nazarov V.B. etc. Preclinical assessment of the effectiveness of local hemostatic drugs (experimental study) // Disaster Medicine. - 2010. - No. 3 (71). — P.23-25.
- Dmitriev V.A. The use of modern local hemostatic agents for severe liver injuries in the system of multi-stage surgical tactics (“DamageControl” for polytrauma (clinical and experimental study): Dissertation... Candidate of Medical Sciences. - St. Petersburg, 2013. - 121 p.
- Miroshnichenko Yu. V., StupnikovA. V., MilyaevA. V. Justification of the composition and structure of the modern system of complete personnel equipment of the military unit of the medical service of the armed forces of the Russian Federation // Bulletin of the Russian Military Medical Academy. — 2011. — T.Z. — pp. 214-219.
- Reva V.A. Rationale for a system for temporarily stopping external bleeding in injuries of the great vessels of the extremities at the prehospital stage: Dissertation....candidate of medical sciences. - St. Petersburg, 2011. - 237 p.
- Samoilov A. S., Vashurina A. O., Lebedev A. O., Borshchevsky V. S. Assessment of the possibility of using the drug “Hemostop” to stop bleeding from liver wounds in conditions of coagulopathy and hypothermia // Proceedings of the interregional scientific and practical conference “Rehabilitation” surgical patients in the context of modernization of domestic and regional healthcare." - 2013. - Chita. — P.140-141.
- Samokhvalov I.M., Reva V.A., Pronchenko A.A. and others. Local hemostatic agents: a new era in the provision of prehospital care // Polytrauma. - 2013. - No. 1. — P.80-82.
- Stepanov Yu. A., Karkishchenko N.N., Cherkasov M. F. et al. Study of the effectiveness of the drug “GEMOSTOP” in an experiment on animals // Biomedicine. - 2010. - No. 5. — P. 50-58.
- Electronic resource: [https:// gosprog.ru]
- Alam N„ Burris D„ DaCortaJ. et al. Hemorrhage control in the battlefield: role of new hemostatic agents // Mil. Med. - 2005. - 170. - P.63-69.
- Alam H„ Chen Z„ Jaskille A. et al. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury // Trauma. — 2004. — 56. — P.974-983.
- Arnaud F., Teranishi K., Tomori T. et al. Comparison of 10 Hemostatic Dressings in a Groin Puncture Model in Swine 11 Vase. Surg. - 2009. - 50. - P.632-639.
- Buddy G. at a!. An Alternative Hemostatic Dressing: Comparison of CELOX, HemCon, and QuikClot 11 Journal Academic Emergency Medicine. - 2008. - 74-81.
- Kheirabadi B., Acheson E., Deguzman R. et al. The potential utility of fibrin sealant dressing in repair of vascular injury in swine // J. Trauma. - 2007. - 62, - P.94-103.
- Kheirabadi ft., McCarron R. et al. Development of a standard swine hemorrhage model for efficacy assessment of topical hemostatic agents//J. Trauma. - 2011. - Vol. 71. - Suppl. 1. — P.139-146.
- Kheirabadi B., Scherer M., Scot J. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine 11 Trauma. - 2009. - 67 - P. 450-460.
- Littlejohn L„ Devlin J., Kircher S. et al. Comparison of Celox-A, ChitoFlex, WoundStat, and Combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in swine model of penetrating trauma // Emerg. Med. - 2011. - Vol. 18. - N4. — P.340-350.