Sodium bicarbonate (baking soda)
Sodium bicarbonate
(English
sodium bicarbonate
; synonyms:
sodium bicarbonate, sodium bicarbonate, baking soda, baking soda
) is an antacid that normalizes acid-base balance.
Sodium bicarbonate is a chemical substance
Sodium bicarbonate is an acidic sodium salt of carbonic acid. Chemical formula of sodium bicarbonate: NaHCO3. Sodium bicarbonate is a white, odorless, crystalline powder with a salty-alkaline taste. Sodium bicarbonate is stable in dry air, but decomposes slowly in humid air. Easily soluble in water to form alkaline solutions. The acidity of a five percent sodium bicarbonate solution = 8.1 pH. Sodium bicarbonate is practically insoluble in ethanol. Molecular weight 84.01. Sodium bicarbonate reacts with acids to form a salt and carbonic acid, which immediately breaks down into carbon dioxide and water.
Sodium bicarbonate - medicine
Sodium bicarbonate (this is its name in pharmaceuticals) is the international nonproprietary name (INN) of the drug. According to the pharmacological index, sodium bicarbonate belongs to the groups “Regulators of water-electrolyte balance and acid-base balance” and “Antacids”, according to ATC - to the group: “B05 Plasma-substituting and perfusion solutions” and has codes B05CB04 and B05XA02. In addition, in the group “A02 Drugs for the treatment of diseases associated with acidity disorders” there is a five-digit code “A02AH Antacids in combination with sodium bicarbonate”.
Sodium bicarbonate therapy for pregnant and nursing mothers
Due to the unwanted side effects of sodium (edema and weight gain), some experts advocate the use of alternative antacids that do not contain sodium bicarbonate in pregnant women. FDA Fetal Risk Category for sodium bicarbonate therapy is "C" (animal studies have shown adverse effects on the fetus and there have been no adequate studies in pregnant women, but the potential benefit associated with the drug in pregnant women may justify its use , despite the existing risk). Due to the lack of data on the excretion of sodium bicarbonate into breast milk, there are no restrictions for the treatment of breastfeeding mothers.
Baking soda is a traditional but dangerous remedy for relieving heartburn.
| All moods and voices Chewed down to one. Enough soda for heartburn! So this is your result, skill? B.L. Parsnip. "All moods and voices." 1936. |
Sodium bicarbonate is a traditional remedy for relieving heartburn, an absorbable antacid. Absorbed antacids are those that either themselves or the products of their reaction with gastric acid dissolve in the blood.
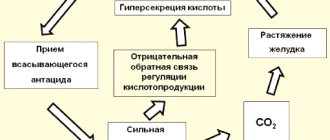
A positive quality of absorbed antacids is the rapid decrease in acidity after taking the medicine. Negative - short duration of action, acid rebound (increased secretion of hydrochloric acid after the end of the drug's effect), the formation of carbon dioxide during their reaction with hydrochloric acid, stretching the stomach and stimulating gastroesophageal reflux (see the figure on the right from the article by D.S. Bordin)
. Absorption of bicarbonates into the blood can lead to the development of systemic alkalosis, and their long-term use in combination with a dairy diet can lead to the development of Burnett's syndrome (milk-alkali syndrome).
After taking 3 g of sodium bicarbonate, the pH of the gastric contents remains above 3 pH units for only 75 minutes. The carbon dioxide formed during neutralization causes belching and bloating. As a rare complication after taking sodium bicarbonate, gastric rupture due to the sudden release of a large amount of gas has been described (A.V. Okhlobystin).
In the 19th century, “soda” was one of the most popular remedies for heartburn; for example, P.I. constantly took it. Chaikovsky.
Sodium bicarbonate in acid-suppressing drugs
Despite the negative attitude towards sodium bicarbonate as a means of relieving heartburn, it is sometimes included in medications intended to reduce acidity in the upper gastrointestinal tract, in sufficient quantities to be considered as another active ingredient (although the instructions for this sodium bicarbonate may be listed among the excipients rather than the active substances).
Proton pump inhibitors
.
There are two versions of the proton pump inhibitor Zegerid
.
Zegerid itself is prescription and over-the-counter Zegerid OTC
.
Zegerid is not registered in Russia, but Omez Insta
. Both medicines contain omeprazole and sodium bicarbonate. In the US, Zegerid is marketed as the only "immediate response" proton pump inhibitor that does not have the "overnight acid breakthrough" phenomenon. It is intended for relief of symptoms of gastroesophageal reflux disease, short-term (4-8 weeks) therapy of erosive esophagitis, confirmed by endoscopy, gastric and duodenal ulcers. The content of omeprazole and sodium bicarbonate in various dosage forms of these drugs is given in the table:
| Main Ingredients | Zegerid , capsule | Zegerid , capsule | Zegerid , sachet* | Zegerid , sachet* | Zegerid OTC , capsule | Omez Insta , sachet* |
| Omeprazole , mg | 20 | 40 | 20 | 40 | 20 | 20 |
| Sodium bicarbonate , mg | 1100 | 1100 | 1680 | 1680 | 1100 | 1680 |
Note.
*) The sachet contains powder for the preparation of a suspension for oral administration. Alginates.
The only medicine for the treatment of acid-related diseases from this group approved in Russia, as well as in the USA, is
Gaviscon
and its variants Gaviscon Forte and Gaviscon Double Action. In addition to the “main” active ingredient - sodium alginate, Gaviscon contains sodium bicarbonate and calcium carbonate. When sodium alginate enters the stomach, it quickly reacts with its acidic contents, resulting in the formation of an alginate gel that has an almost neutral acidity value (gel pH is about 7). The gel forms a protective barrier on the surface of the stomach contents, preventing the occurrence of gastroesophageal reflux. In case of regurgitation or reflux, the gel enters the esophagus, where it has a neutralizing effect on hydrochloric acid and pepsin that enter during reflux and additionally protects the mucous membrane of the esophagus. The content of sodium alginate and sodium bicarbonate in various dosage forms of Gaviscon is given in the table:
| Main Ingredients | Gaviscon , 10 ml suspension | Gaviscon , chewable tablet | Gaviscon forte , 10 ml suspension | Gaviscon Double Action , chewable tablet | Gaviscon Double Action , 10 ml suspension |
| Sodium alginate , mg | 500 | 250 | 1000 | 250 | 500 |
| Sodium bicarbonate , mg | 267 | 133,5 | — | 267 | 213 |
Sodium bicarbonate in other medicines
In addition to the medications described above for the treatment of acid-dependent diseases, sodium bicarbonate is included as an active ingredient, in particular, in the following medications:
- osmotic laxative Endofalk
, one sachet of which includes: macrogol 3350 52.5 g, sodium chloride 1.4 g, sodium bicarbonate 715 mg, potassium chloride 185 mg - osmotic laxative Lavacol
, one sachet of which includes: macrogol 4000 12.0 g, sodium sulfate anhydrous 1.0 g, sodium bicarbonate 0.6 g, sodium chloride 0.2 g, potassium chloride 0.2 g - antitussive drug Codelac
, one tablet of which contains: codeine 8 mg, sodium bicarbonate 200 mg, licorice root powder 200 mg, thermopsis lanceolate herb powder 20 mg - a drug for the treatment of cough that has a mucolytic and expectorant effect, Codelac Broncho
, one tablet of which contains: ambroxol hydrochloride 20 mg, sodium glycyrrhizinate 30 mg, thermopsis dry extract 10 mg, sodium bicarbonate 200 mg - solution for infusion with detoxification effect Gemodez-N
, 100 ml of which contains: medical povidone with a molecular weight of 8000 6 g, sodium chloride 550 mg, potassium chloride 42 mg, calcium chloride 50 mg, magnesium chloride anhydrous 500 mcg, sodium bicarbonate 23 mg
The use of sodium bicarbonate in the study of the stomach (Noller test)
The Knoller test (alkaline test) is performed to obtain information about the amount of hydrochloric acid in the patient’s stomach, the intensity of acid formation, and also, indirectly, the amount of gastric juice. The test is carried out simultaneously with the intragastric pH-metry
, 20 minutes after stabilization of acidity under basal conditions or 45 minutes after the administration of stimulants. At a pH equal to or higher than 4.0, the alkaline test is not performed.
The patient drinks 0.5 g of sodium bicarbonate dissolved in 30 ml of distilled water. Typically, the pH in the body of the stomach is recorded below 2.5. As a result of the introduction of alkali into the stomach, the pH values change to alkaline and remain at the same level for a certain time, and then, after some time, called “alkaline time,” they return to the original ones. The alkaline time determines the state of acid production in the patient’s stomach (S.I. Rapoport et al.):
| Assessment of hydrochloric acid production in the stomach | Alkaline time , min | |
| on an empty stomach | upon stimulation | |
| Sharply increased acid production | <10 | <5 |
| Increased acid production | 10–20 | 5–10 |
| Normal acid production | 20–25 | 10–15 |
| Reduced acid production | >25 | >15 |
The figure shows an example of a pH gram in
three sections of the stomach
(in the antrum - lower graph, in the body of the stomach - middle and in the cardiac section of the stomach closest to the esophagus - lower graph) of a patient with chronic superficial gastritis and high acidity in the antrum. Basal acidity is shown (the first 30 minutes, basal - that is, before any stimulation) and acidity after the alkaline test (AL) and histamine stimulation (HT).
Bicarbonates as a natural means of protecting the gastrointestinal tract from acid
In the stomach and duodenum, bicarbonate ions HCO3– are secreted by surface epithelial cells. Bicarbonates play a vital role in the digestive process, neutralizing hydrochloric acid and protecting the tissues of the digestive organs from its effects.
In an acidic environment, bicarbonate ions react irreversibly with hydrogen ions to form water and carbon dioxide:
H+ + HCO3– = H2CO3 = H2O + CO2
Bicarbonates, together with mucus, constitute the so-called pre-epithelial level of protection of the gastric mucosa. Mucus cannot protect the epithelium from H+ ions without bicarbonates constantly entering it, also secreted by the surface epithelium. With the help of bicarbonates, a pH gradient is maintained in the mucus: on the surface facing the lumen of the stomach, the environment is acidic, and on the epithelial cells it is neutral or slightly alkaline. Immediate mixing of bicarbonates with the acidic secretion in the lumen and neutralization does not occur: the mucus layer forms a barrier, due to which the pH gradient exists (T.L. Lapina).
Bicarbonates are also secreted by the ductal cells of the pancreas and, together with pancreatic juice, enter the duodenum, where they participate in the neutralization of hydrochloric acid (O.A. Sablin et al.).
Bicarbonate in mineral waters
Hydrocarbonate ions HCO3– are present in almost all natural mineral waters.
To determine them in water, GOST 23268.3-78 “Healing mineral drinking waters, medicinal table waters and natural table waters” is used. Methods for determining bicarbonate ions". According to GOST R 54316-2011. “Natural drinking mineral waters. General technical conditions" the content of bicarbonates is indicated on consumer packaging (on the labels of bottles of mineral water). Content of hydrocarbonates in some natural mineral waters (g/l):
- healing mineral waters:
- Nagutskaya-17 — 5.0–7.2
- Essentuki No. 17 — 4.9–6.5
- Nagutskaya-56 — 4.2–5.6
- Essentuki No. 4 - 3.4–4.8
- Narzan - 1.0–1.5
- Kashinskaya - less than 0.05
- Gelendzhikskaya 117 — 0.35–0.7
- Essentuki Novaya-55 - 0.2–0.35
Bicarbonate anions give mineral water an alkaline character and most often combine with sodium cations to form sodium bicarbonate. Sodium bicarbonate waters increase the alkaline reserve of the blood, have an antacid effect (by reducing the concentration of H+ ions), reduce pyloric spasm and accelerate the evacuation of gastric contents, which helps reduce pain and dyspeptic symptoms. Alkaline waters thin and help remove excess mucus, which is formed during inflammation in the gastrointestinal tract, urinary and respiratory tracts. In addition, they improve nucleic acid metabolism, reduce the formation of uric acid and promote the removal of excess from the body, alkalize bile and increase the secretion of bilirubin, cholesterol and mucus. In diabetes mellitus, these waters reduce hyperglycemia and increase tolerance to carbohydrates. And finally, in combination with bicarbonate, macro- and microelements, in particular iron, are better absorbed from the intestine (Baranovsky A.Yu. et al.).
Professional medical articles addressing the role of bicarbonates and sodium bicarbonate in gastroenterology
- Mikheev A.G., Nevsky D.I., Rakitin A.B., Rakitin B.V. Study of pH dynamics in alkaline dough // Izvestiya VUZov. North Caucasus region. Natural Sciences. – 2006. Special issue. – pp. 44–46.
- Sablin O.A., Grinevich V.B., Uspensky Yu.P., Ratnikov V.A. Stomach. Methods for studying alkaline secretion. Functional diagnostics in gastroenterology. St. Petersburg 2002
- Lapina T.L. Possibilities of medicinal effects on the cytoprotective properties of the gastroduodenal mucosa // Russian Journal of Gastroenterology, Hepatology, Coloproctology. – 2006. – No. 5. – volume XVI. – c. 2–7.
- Ushkalova E.A. Clinical pharmacology of modern antacids // Farmateka. – 2006. – No. 11. – p.1–6.
- Gelfand B.R. , Filimonov M.I., Mamontova O.A. and others. Prevention and treatment of stress damage to the gastrointestinal tract in patients in critical conditions // Methodological recommendations. - Moscow. - 2010. - 34 p.
On the website in the literature catalog there is a section “Antacids”, containing articles devoted to the treatment of diseases of the gastrointestinal tract with antacids. Back to section
Sodium bicarbonate solution for infusion 40 mg/ml in 100 ml glass bottle No. 1
Name
Sodium bicarbonate solution dinf.40 mgml in bottle glass.100ml in pack No. 1
Description
Colorless transparent solution.
Main active ingredient
Sodium bicarbonate
Release form
solution for infusion
Dosage
40 mg / 1 ml 100 ml
special instructions
Not applicable; sodium bicarbonate is intended for use only in urgent situations.
pharmachologic effect
Plasma replacement and perfusion solutions. Electrolyte solutions. ATS code. B05ХА02.
Pharmacodynamics
A remedy for restoring the alkaline state of the blood and correcting metabolic acidosis. When sodium bicarbonate dissociates, a bicarbonate anion is released, which binds hydrogen ions to form a carboxylic acid, which then breaks down into water and exhaled carbon dioxide. As a result, the blood pH shifts to the alkaline side, and the buffer capacity of the blood increases. The drug also increases the excretion of sodium and chloride ions from the body, increases osmotic diuresis, and alkalinizes urine, preventing the precipitation of uric acid in the urinary system. Bicarbonate anion does not penetrate into cells
Pharmacokinetics
Sodium bicarbonate in water dissociates into sodium ions (Na+) and bicarbonate ions (HCO3-). Sodium (Na+) is the main cation in the extracellular fluid, bicarbonate is a key component of the bicarbonate buffer system of the blood. When relatively large amounts of acidic products are released into the blood, hydrogen ions H+ interact with HCO3-, which leads to the formation of weakly dissociating carbonic acid - H2CO3. Under the action of the enzyme carbanhydrase, carbonic acid is broken down to form CO2, which is removed with exhaled air and H2O. The concentration of bicarbonate ions in plasma is regulated by the kidney through acidification of urine in case of HCO3 deficiency or alkalinization of urine in case of excess.
Indications for use
It is used to correct decompensated metabolic acidosis in various diseases and conditions (the absolute indication is a decrease in blood pH below 7.2).
Directions for use and doses
Administer intravenously to adults and children, depending on the severity of acidosis. Apply undiluted or diluted 5% glucose solution in a 1:1 ratio. Formula for calculating the volume of sodium bicarbonate administered: amount (in ml) of 4% sodium bicarbonate = 0.2 x BE x M (kg); where BE is base deficiency, M is body weight, 0.2 is the calculation of the extracellular space of the body. The usual dose is 1 mmol/kg followed by 0.5 mmol/kg at 10 minute intervals. It is recommended to first introduce half the calculated amount of sodium bicarbonate in order to be able to adjust the initially calculated amount after re-determining the acid-base balance. Half of the calculated amount of solution is administered intravenously in a slow stream, the rest - dropwise at a rate of 60 drops per minute. The maximum dose for adults is 300 ml per day, for children from 100 to 200 ml depending on body weight. The total amount of sodium bicarbonate should be administered to older children and adults over a 4-8 hour period under the control of the acid-base balance of the blood and the clinical condition of the patient. Sodium bicarbonate therapy should be gradual, since the clinical response to the administered dose is not always predictable.
Use during pregnancy and lactation
The safety of sodium bicarbonate during pregnancy in humans has not been established. The use of a drug during pregnancy is possible if the expected benefit to the mother outweighs the possible risk to the fetus. Due to the sodium content of the drug, special care should be taken in case of eclampsia. There is no data on the penetration of sodium bicarbonate into breast milk. The use of the drug during breastfeeding is possible if the expected benefit to the mother outweighs the possible risk to the child.
Precautionary measures
Before administration, conduct a visual inspection of the bottle, check the tightness of the packaging and the presence of the label. The solution must be clear and free of suspended particles or sediment. Sodium bicarbonate should not be administered as a stream. Complications of rapid jet administration: increased blood osmolarity, hypernatremia, risk of intracranial hemorrhage (ICH) and respiratory arrest, worsening heart failure, pronounced fluctuations in blood pressure and cerebral blood flow, increased intracellular acidosis and cerebral edema, development of hypokalemia and tissue hypoxia due to impaired dissociation of oxyhemoglobin. When using the drug, it is necessary to monitor the acid-base state of the blood. Patients with concomitant heart or kidney disease may develop heart failure and edema. A shift in the acid-base state of the blood to the alkaline side during chronic renal failure (CKF) can significantly worsen the patient’s clinical condition. When metabolic acidosis is accompanied by respiratory acidosis, pulmonary ventilation and drug infusion must be properly controlled to prevent an increase in blood PaCO2 and, as a consequence, an increase in cerebral acidosis. Accidental extravascular administration of the drug solution should be avoided (see section “Adverse reactions”). Newborns (including premature infants) and children under 2 years of age: rapid infusion (up to 1 ml/min) of hypertonic sodium bicarbonate solutions can lead to hypernatremia with the development of intracranial hemorrhage. It is necessary to control the water and electrolyte balance!
Interaction with other drugs
Use with caution in patients receiving corticosteroids and corticotropin. With the simultaneous administration of sodium bicarbonate, due to an increase in urine pH, the excretion by the kidneys of drugs that are acidic in nature, for example, tetracyclines, especially doxycycline, acetylsalicylic acid, chlorpropamide, lithium, urotropine, may increase. Sodium bicarbonate may increase the half-life and duration of action of base drugs such as quinidine, amphetamines, ephedrine, memantine and flecainide. When used simultaneously with methotrexate, its excretion in the urine increases and its toxic effect on the kidneys decreases due to an increase in urine pH under the influence of sodium bicarbonate. Hypochloremic alkalosis may occur if sodium bicarbonate is used in combination with non-potassium sparing diuretics such as ethacrynic acid, thiazides and furosemide. Concomitant use in patients taking potassium supplements may decrease serum potassium by activating intracellular potassium ion shift. The addition of sodium bicarbonate to parenteral solutions containing calcium should be avoided. Sodium bicarbonate is not compatible with solutions of: ascorbic, nicotinic and other acids; alkaloids (atropine, apomorphine, caffeine, theobromine, papaverine, etc.), cardiac glycosides, calcium, magnesium salts, heavy metals (iron, copper, zinc), because Precipitation or hydrolysis of organic compounds occurs. Do not mix with phosphate-containing solutions. Due to physical incompatibility (change in color, transparency, formation of microparticles, sediment, bubbles) and/or chemical instability, the administration of sodium bicarbonate is incompatible with: allopurinol sodium, amiodarone hydrochloride, amoxicillin sodium, amphotericin, ampicillin sodium, anidulafungin, anileridine, ascorbic acid solution acid, atracuria besylate, azacidite, azathioprine sodium, buprenorphine hydrochloride, butorphanol tartrate, calcium chloride, calcium gluconate, carboplatin, carmustine, caspofungin, cefamandole, cefotaxime, cefotetan disodium salt, cefuroxime, chlorpromazine hydrochloride, ciprofloxacin , cisplatin, clonazepam, codeine phosphate, dantrolene sodium, daunorubicin citrate, diazepam, diphenhydramine hydrochloride, diazoxide, dimenhydrinate, dobutamine hydrochloride, dopamine hydrochloride, doxapram, doxorubicin hydrochloride, doxycycline, adrenaline hydrochloride, epirubicin hydrochloride, sodium ertapenem, esmolol hydrochloride , etidocaine hydrochloride, phenoldopamine mesylate, glycopyrrolate, haloperidol lactate , ganciclovir sodium, hydromorphone hydrochloride, hydroxyzine hydrochloride, idarucibin hydrochloride, imipenem with cilastatin, isoproterenol hydrochloride, ketamine hydrochloride, labetalol hydrochloride, lansoprazole, leucovorin calcium, levobupivacaine hydrochloride, levofloxacin, levorphanol tartrate , magnesium sulfate, hydromorphone hydrochloride, meropenem, methadone, metoclopramide hydrochloride, midazolam hydrochloride, minocycline hydrochloride, morphine sulfate, moxalactam, mycophenolate mofetil hydrochloride, nalbuphine hydrochloride, nicardipine hydrochloride, norepinephrine bitartrate, ondansetron hydrochloride, oxytetracycline, pantoprazole sodium, papaverine hydrochloride, pentamidine, pen tazocin lactate, sodium pentobarbital, sodium phenytoin, procaine hydrochloride, prochlorperazine edisylate, promethazine hydrochloride, propacetamol, quinidine gluconate, rituximab, secobarbital, sodium lactate, streptomycin sulfate, succinylcholine chloride, sulfamethoxazole with trimethoprim, tetracycline, thiamine hydrochloride, sodium thiopental, ticarcillin with clav ulanic acid, trimethaphan, tubocurarine, verapamil hydrochloride, vindesine sulfate , vinorelbine tartrate, B complex vitamins with vitamin C, and voriconazole. Sodium bicarbonate is also incompatible with solutions: 5% alcohol in 5% dextrose, 5% dextrose in lactated Ringer's solution.
Contraindications
Metabolic or respiratory alkalosis, hypokalemia, hypernatremia, hypoventilation, hypochloremia, hypocalcemia, conditions accompanied by hyperosmolarity, congestive heart failure, renal failure, history of kidney stones, edema, arterial hypertension, eclampsia.
Compound
| Sodium bicarbonate | 4 g (47.6 mmol) | 12 g (142.9 mmol) |
| Water for injections | up to 100 ml | up to 300 ml |
| Theoretical osmolality | 762 mOsmol/kg | 762 mOsmol/kg |
| pH | 8,1-8,9 | 8,1-8,9 |
Overdose
If the dose of the drug is exceeded, hyperalkalosis, hypernatremia and hyperosmia, and tetanic convulsions may develop. If signs of alkalosis appear (convulsions, including with manifestations of tetany, agitation, decreased potassium and calcium levels, increased sodium levels in the blood, increased pH levels), stop administering the drug; if there is a risk of developing convulsions, administer calcium gluconate intravenously; in case of hypokalemia potassium supplements are administered.
Side effect
Metabolic and nutritional disorders: alkalosis, hypokalemia, hypernatremia, hyperosmolarity, hypocalcemia, hypoglycemia, paradoxical intracellular acidosis. Cardiovascular system disorders: deterioration of hemodynamics associated with volume overload, increased blood pressure. Nervous system disorders: intracranial hemorrhage (in newborns), headache, irritability, tetany. Gastrointestinal disorders: loss of appetite, nausea, vomiting, abdominal pain. General disorders and disorders at the injection site: improper administration (intraarterial, paravenous) can lead to tissue necrosis.
Storage conditions
At temperatures from 15 °C to 25 °C. Note. Non-wetting of the inner surface of the bottles is not a contraindication to the use of the drug.