Pharmacodynamics and pharmacokinetics
Metformin is a biguanide with a hypoglycemic effect that can reduce the concentration of glucose in the blood plasma. However, it does not stimulate the production of insulin , and therefore does not cause hypoglycemia . During treatment, peripheral receptors become more sensitive to insulin, and glucose utilization by cells increases. The synthesis of glucose by the liver is reduced due to inhibition of glycogenolysis and gluconeogenesis. There was a delay in glucose absorption in the gastrointestinal tract.
The active component of the drug stimulates the production of glycogen by acting on glycogen synthase. The transport capacity of any membrane glucose transporters increases.
When treated with metformin, patients maintain body weight or notice a moderate decrease. The substance has a beneficial effect on lipid metabolism: reducing the level of total cholesterol, triglycerides and LDL.
Extended-release tablets are characterized by slow absorption. Therefore, the therapeutic effect lasts for at least 7 hours. The absorption of the drug does not depend on food and does not cause accumulation. Slight binding to plasma proteins is noted. Metabolism occurs without the formation of metabolites. The components are excreted unchanged through the kidneys.
Glucophage Long, prolong tablets. 1000 mg, 60 pcs.
Lactic acidosis
Lactic acidosis is a rare but serious (high mortality unless promptly treated) complication that may occur due to accumulation of metformin. Cases of lactic acidosis when taking metformin occurred mainly in diabetic patients with severe renal failure.
Other associated risk factors should be taken into account, such as decompensated diabetes mellitus, ketosis, prolonged fasting, alcoholism, liver failure, any condition associated with severe hypoxia, severe infectious disease and concomitant use with certain medications (see “Interaction”). This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspeptic disorders, abdominal pain and severe asthenia.
Lactic acidosis is characterized by severe malaise with general weakness, acidotic shortness of breath and vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (less than 7.35), plasma lactate concentration over 5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function has been found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment and regularly thereafter, it is necessary to determine creatinine Cl at least once a year in patients with normal renal function and 2-4 times a year in elderly patients, as well as in patients with creatinine Cl on NGN.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients, simultaneous use of antihypertensive drugs, diuretics or NSAIDs, dehydration (chronic or severe diarrhea, repeated bouts of vomiting).
Heart failure
Patients with heart failure have a higher risk of developing hypoxia and renal failure. Patients with CHF should regularly monitor cardiac and renal function while taking metformin. Taking metformin in acute heart failure and CHF with unstable hemodynamic parameters is contraindicated.
Other Precautions
Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly.
Patients should inform their doctor about any treatment they are undergoing and any infectious diseases such as colds, respiratory or urinary tract infections.
It is recommended that routine laboratory tests be performed regularly to monitor diabetes mellitus.
Metformin does not cause hypoglycemia when used alone, but caution is recommended when used in combination with insulin or other oral hypoglycemic agents (for example, sulfonylureas or repaglinide). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, and difficulty seeing or concentrating.
It is necessary to warn the patient that the excipients of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the pharmacological effect of the drug.
Impact on the ability to drive vehicles and machinery.
Monotherapy with Glucophage® Long does not cause hypoglycemia and therefore does not affect the ability to drive vehicles and machines. However, the development of hypoglycemia is possible when metformin is used in combination with other hypoglycemic drugs (for example, sulfonylureas, insulin, repaglinide). If symptoms of hypoglycemia appear, you should not drive vehicles or machinery.
Contraindications for use
The drug is not prescribed for:
- sensitivity to metformin and other components;
- diabetic ketoacidosis , precoma , coma ;
- impairment or insufficiency of kidney or liver function;
- acute forms of various diseases;
- extensive injuries and operations;
- chronic alcoholism , alcohol intoxication;
- pregnancy;
- lactic acidosis;
- use 48 hours before or after radioisotope or x-ray studies involving the administration of iodinated contrast agent;
- hypocaloric diets;
- under 18 years of age.
Caution when prescribing this drug should be exercised in relation to elderly patients and people performing heavy physical work, as this can cause the development of lactic acidosis when treating nursing women.
Side effects
During drug therapy, the development of lactic acidosis , megaloblastic anemia , and decreased absorption of vitamin B12 is possible.
Disturbances in the functioning of the nervous system and gastrointestinal tract are also possible: changes in taste, nausea, vomiting, pain, diarrhea, loss of appetite. Typically, such symptoms bother you at the beginning of treatment and gradually disappear. To prevent their development, patients are recommended to take metformin together or immediately after meals.
In rare cases, deviations in the activity of the liver and bile and the manifestation of allergic skin reactions .
Glucophage Long tab prolong 500 mg N30 (Merck)
Lactic acidosis Lactic acidosis is an extremely rare but serious (high mortality in the absence of immediate treatment) complication that can occur due to the accumulation of metformin. Cases of lactic acidosis in patients receiving metformin have occurred primarily in diabetic patients with advanced renal impairment. Other associated risk factors should be considered, such as poorly controlled diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis. The risk of developing lactic acidosis should be considered if nonspecific signs appear, such as muscle cramps accompanied by dyspepsia, abdominal pain, general weakness and severe malaise. Lactic acidosis is characterized by acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (5 mmol/l, increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately. Surgeries Metformin use should be discontinued 48 hours before planned surgical operations and can be continued no earlier than 48 hours after, provided that during the examination, renal function was found to be normal. Renal function Since metformin is excreted by the kidneys, before starting treatment, and regularly thereafter, it is necessary to determine CC: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with CK at the lower limit of normal. Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with the simultaneous use of antihypertensive drugs, diuretics or NSAIDs. Other precautions Patients are recommended to continue to follow a diet with even carbohydrate intake throughout the day. Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly. Patients should tell their doctor about any treatment they are undergoing and any infectious diseases, such as respiratory or urinary tract infections. Standard laboratory tests should be performed regularly to monitor diabetes mellitus. Metformin alone does not cause hypoglycemia, however Caution is recommended when used in combination with insulin or other oral hypoglycemic agents (eg, sulfonylureas or repaglinide). Symptoms of hypoglycemia are weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision or impaired concentration. The patient must be warned that the inactive components of Glucophage Long may be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug. Effect on the ability to drive vehicles and operate machinery Monotherapy with Glucophage® Long does not cause hypoglycemia, and therefore does not affect the ability to drive a car or operate machinery. However, patients should be warned about the risk of hypoglycemia when using metformin in combination with other hypoglycemic drugs ( sulfonylurea derivatives, insulin, repaglinide).
Instructions for use Glucophage Long (Method and dosage)
The tablets are intended to be taken orally whole with a small volume of liquid. It is recommended to do this daily during dinner.
According to the instructions for use, the choice of dosage of extended-release tablets is carried out individually for a particular patient, taking into account blood glucose concentrations.
Glucophage Long 750 mg and 500 mg can be prescribed as mono- or combination therapy. It is important to strictly adhere to the prescribed dosages and regularly monitor blood sugar levels.
Glucophage Long, 30 pcs., 1 g, extended-release tablets
Inside.
The tablets are swallowed whole, without chewing, with a small amount of liquid, 1 time per day during dinner. The dose of the drug Glucophage® Long in the form of extended-release tablets is selected by the doctor individually for each patient based on the results of measuring the concentration of glucose in the blood.
Monotherapy and combination therapy in combination with other hypoglycemic agents.
For patients not taking metformin, the recommended starting dose of Glucophage Long is 500, 750 or 1000 mg once daily with dinner.
500 mg. Depending on the concentration of glucose in the blood plasma, every 10–15 days the dose can be slowly increased (by 500 mg) until the maximum daily dose is reached (2000 mg). Slowly increasing the dose helps reduce gastrointestinal side effects.
750 mg. The recommended dose of Glucophage® Long is 2 tablets. 750 mg 1 time per day. If, when taking the recommended dose, it is not possible to achieve adequate glycemic control, it is possible to increase the dose to the maximum - 3 tablets. 750 mg of Glucophage® Long 1 time per day.
1000 mg. Glucophage Long 1000 mg is prescribed as maintenance therapy for patients taking metformin in the form of regular-release tablets at a dose of 1000 or 2000 mg.
500, 750 and 1000 mg. For patients already receiving treatment with metformin, the initial dose of Glucophage Long should be equivalent to the daily dose of regular-release tablets.
Patients taking metformin in the form of regular-release tablets at a dose exceeding 2000 mg are not recommended to switch to Glucophage Long.
For patients not taking metformin, the recommended starting dose of Glucophage®Long is 500 mg or 750 mg once daily with dinner (the following formulations of Glucophage®Long are available: extended-release tablets 500 mg and 750 mg). Every 10–15 days, it is recommended to adjust the dose based on the results of measuring blood glucose concentrations.
In case of switching from another hypoglycemic agent, dose selection is carried out as described above, starting with the administration of the drug Glucophage® Long 500 or 750 mg, with a possible subsequent transition to the drug Glucophage® Long 1000 mg.
Combination with insulin.
To achieve better blood glucose control, metformin and insulin can be used in combination therapy. The usual starting dose of Glucophage® Long is 1 tablet. 500 or 750 mg once daily with dinner, while the insulin dose is adjusted based on blood glucose measurements. Then it is possible to switch to the drug Glucophage® Long 1000 mg.
Daily dose.
The maximum recommended dose of Glucophage® Long is 4 tablets. 500 mg (2000 mg/day), 3 tablets. 750 mg per day (2250 mg) or 2 tablets. 1000 mg per day (2000 mg). If, when taking the maximum recommended dose once a day during dinner, it is not possible to achieve adequate glycemic control, then the maximum dose can be divided into two doses: 2 tablets. 500 mg or 1 tablet. 1000 mg - during breakfast and 2 tablets. 500 mg or 1 tablet. 1000 mg - during dinner.
If adequate glycemic control is not achieved when taking 2000 mg of Glucophage® Long, extended-release tablets, a switch to metformin with regular release of the active ingredient (for example, Glucophage®, film-coated tablets) with a maximum daily dose of 3000 mg is possible.
Patients with renal failure.
Metformin can be used in patients with moderate renal failure (Cl creatinine 45–59 ml/min) only in the absence of conditions that may increase the risk of developing lactic acidosis. The initial dose is 500 or 750 mg 1 time per day. The maximum dose is 1000 mg/day. Renal function should be carefully monitored every 3–6 months.
If creatinine Cl is below 45 ml/min, the drug should be stopped immediately.
Elderly patients.
Due to a possible decrease in renal function, the dose of metformin is adjusted based on an assessment of renal function, which should be carried out regularly, at least 2 times a year.
Duration of treatment.
Glucophage® Long should be taken daily, without interruption. If treatment is stopped, the patient must inform the doctor.
Missing a dose.
If a dose is missed, the patient should take the next dose at the usual time. You should not take a double dose of Glucophage® Long.
Overdose
Taking metformin in a dosage of less than 85 g does not cause hypoglycemia . But the possibility of developing lactic acidosis remains.
If symptoms of lactic acidosis appear, you must immediately stop taking the medication and determine the lactate concentration in a hospital setting to clarify the diagnosis. The effectiveness of the procedure for removing lactate and metformin from the body using hemodialysis has been noted. Concomitant symptomatic therapy is also carried out.
Interaction
The development of lactic acidosis can be caused by a combination of the drug with iodine-containing radiocontrast agents. Therefore, 48 hours before and after radiological examination using iodine-containing radiopaque agents, it is recommended to discontinue Glucophage Long.
Concomitant use with drugs with an indirect hyperglycemic effect - hormonal agents or tetracosactide , as well as β2-adrenergic agonists, danazol, Chlorpromazine and diuretics can affect the concentration of glucose in the blood. Therefore, it is necessary to monitor its indicators, and, if necessary, adjust dosages.
In addition, in the presence of renal failure, diuretics contribute to the development of lactic acidosis . Combination with sulfonylurea derivatives , acarbose , insulin , and salicylates often causes hypoglycemia.
Combinations with amiloride , digoxin , morphine , procainamide , quinidine , quinine , ranitidine , triamterene , trimethoprim and vancomycin , which are secreted in the renal tubules, compete with metformin for tubular transport, which increases its concentration.
Glucophage Long tablet prolong 750 mg pack cont cell/pack card x30
ATX code: A10BA02 (Metformin) Active substance: metformin (metformin) Rec.INN registered by WHO Dosage form GLUCOPHAGE® LONG tab. prolong. action 750 mg: 30 or 60 pcs. reg. No.: LP-000509 dated 03/01/11 - Valid Release form, composition and packaging Extended-release tablets are white or almost white, capsule-shaped, biconvex, engraved “750” on one side and “Merck” on the other.
1 tab. metformin hydrochloride 750 mg
Excipients: carmellose sodium - 37.5 mg, hypromellose 2208 - 294.24 mg, magnesium stearate - 5.3 mg.
15 pcs. - blisters (2) - cardboard packs. 15 pcs. - blisters (4) - cardboard packs.
Clinical-pharmacological group: Oral hypoglycemic drug Pharmaco-therapeutic group: Hypoglycemic agent for oral administration of the biguanide group
Indications Treatment of type 2 diabetes mellitus in adults with ineffective diet therapy and physical exercise (especially in obese patients):
- as monotherapy,
- in combination with other oral hypoglycemic drugs, or in combination with insulin.
ICD-10 codes ICD-10 code Indication E11 Non-insulin-dependent diabetes mellitus (type 2 diabetes mellitus)
Dosage regimen The drug is taken orally 1 time/day, during dinner. The tablets are swallowed whole, without chewing, with a sufficient amount of liquid.
The dose of Glucophage Long should be selected individually for each patient based on the results of measuring blood glucose concentrations.
Glucophage® Long should be taken daily, without interruption. If treatment is stopped, the patient must inform the doctor.
If you miss a dose, the next dose should be taken at the usual time. You cannot double the dose of Glucophage® Long.
Monotherapy and combination therapy in combination with other hypoglycemic agents
For patients not taking metformin, the recommended initial dose of Glucophage® Long is 1 tablet. 1 time/day
Every 10-15 days of treatment, it is recommended to adjust the dose based on the results of measuring blood glucose concentrations. Slowly increasing the dose helps reduce gastrointestinal side effects.
The recommended dose of Glucophage® Long is 1500 mg (2 tablets) 1 time/day. If, when taking the recommended dose, it is not possible to achieve adequate glycemic control, it is possible to increase the dose to the maximum - 2250 mg (3 tablets) 1 time / day.
If adequate glycemic control is not achieved when taking 3 tablets. 750 mg 1 time/day, then it is possible to switch to a metformin preparation with a regular release of the active substance (for example, Glucophage®, film-coated tablets) with a maximum daily dose of 3000 mg.
For patients already receiving treatment with metformin tablets, the initial dose of Glucophage Long should be equivalent to the daily dose of regular-release tablets. Patients taking metformin in the form of regular-release tablets at a dose exceeding 2000 mg are not recommended to switch to Glucophage Long.
If you are planning a transition from another hypoglycemic agent: you must stop taking the other drug and start taking Glucophage® Long at the dose indicated above.
Combination with insulin
To achieve better control of blood glucose concentrations, metformin and insulin can be used in combination therapy. The usual starting dose of Glucophage® Long is 1 tablet. 750 mg 1 time/day during dinner, with the dose of insulin selected based on the results of measuring blood glucose levels.
In elderly patients and patients with reduced renal function, the dose is adjusted based on an assessment of renal function, which must be carried out regularly, at least 2 times a year.
Side effects Determination of the frequency of side effects: very often (≥1/10), often (≥1/100, From the nervous system: often - taste disturbance (metallic taste in the mouth).
From the digestive system: very often - nausea, vomiting, diarrhea, abdominal pain, lack of appetite. Most often they occur during the initial period of treatment and in most cases resolve spontaneously. To prevent symptoms, it is recommended to take metformin with meals. Slowly increasing the dose may improve gastrointestinal tolerability.
From the hepatobiliary system: very rarely - abnormal liver function tests or hepatitis; after discontinuation of metformin, adverse effects completely disappear.
From the skin: very rarely - erythema, itching, urticaria.
Metabolic disorders: very rarely - lactic acidosis. Long-term use of metformin may reduce the absorption of vitamin B12. When megaloblastic anemia is detected, the possibility of such an etiology must be taken into account.
If any of the side effects indicated in the instructions are aggravated, or other side effects not listed in the instructions are noted, the patient should inform the doctor.
Contraindications for use: diabetic ketoacidosis, diabetic precoma, coma,
- renal failure or impaired renal function (KK - acute conditions that occur with a risk of developing renal dysfunction, including dehydration (with chronic or severe diarrhea, repeated bouts of vomiting), severe infectious diseases (for example, respiratory and urinary tract infections ), shock,
- clinically pronounced manifestations of acute and chronic diseases that can lead to the development of tissue hypoxia (including cardiac or respiratory failure, acute myocardial infarction),
- extensive surgical operations and injuries, when insulin therapy is indicated,
- liver failure, liver dysfunction,
- chronic alcoholism, acute alcohol poisoning,
- lactic acidosis (including history),
- use for at least 48 hours before and 48 hours after radioisotope or x-ray studies with the introduction of an iodine-containing contrast agent,
— adherence to a hypocaloric diet (less than 1000 kcal/day),
- pregnancy,
- children and adolescents under 18 years of age due to the lack of data on use,
- hypersensitivity to the components of the drug.
The drug should be used with caution in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis during lactation (breastfeeding).
Use during pregnancy and lactation Decompensated diabetes mellitus during pregnancy is associated with an increased risk of birth defects and perinatal mortality.
Limited evidence suggests that the use of metformin in pregnant women does not increase the risk of birth defects in children.
When planning pregnancy, as well as in case of pregnancy while using metformin, the drug should be discontinued and insulin therapy should be prescribed. It is necessary to maintain blood glucose concentrations at a level as close to normal as possible to reduce the risk of fetal malformations.
Metformin is excreted in breast milk. No side effects were observed in breastfeeding newborns while taking metformin. However, due to limited data, the use of the drug during breastfeeding is not recommended. The decision to stop breastfeeding should be made taking into account the benefits of breastfeeding and the potential risk of side effects in the baby.
Use for liver dysfunction Contraindication: liver failure, liver dysfunction. Use for impaired renal function Contraindicated in case of renal failure or impaired renal function (creatinine clearance less than 60 ml/min), in acute conditions that occur with a risk of developing impaired renal function, incl. dehydration (with chronic or severe diarrhea, repeated bouts of vomiting), severe infectious diseases (for example, respiratory and urinary tract infections), shock.
Use in children Contraindicated in children and adolescents under 18 years of age due to the lack of data on use.
Use in elderly patients The drug should be used with caution in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis. Special instructions Lactic acidosis
Lactic acidosis is an extremely rare but serious (high mortality rate unless promptly treated) complication that can occur due to accumulation of metformin. Cases of lactic acidosis in patients receiving metformin occurred mainly in diabetic patients with severe renal failure.
Other associated risk factors should be considered, such as poorly controlled diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with severe hypoxia. This may help reduce the incidence of lactic acidosis.
The risk of developing lactic acidosis should be taken into account when nonspecific signs appear, such as muscle cramps accompanied by dyspepsia, abdominal pain, general weakness and severe malaise.
Lactic acidosis is characterized by acidotic shortness of breath, vomiting, abdominal pain, muscle cramps and hypothermia followed by coma. Diagnostic laboratory parameters are a decrease in blood pH (5 mmol/l), increased anion gap and lactate/pyruvate ratio. If lactic acidosis is suspected, stop taking the drug and consult a doctor immediately.
Surgical operations
The use of metformin should be discontinued 48 hours before elective surgery and can be continued no earlier than 48 hours after, provided that renal function has been found to be normal during the examination.
Kidney function
Since metformin is excreted by the kidneys, before starting treatment, and regularly thereafter, it is necessary to determine CC: at least once a year in patients with normal renal function, and 2-4 times a year in elderly patients, as well as in patients with CC on lower limit of normal.
Particular caution should be exercised in case of possible impairment of renal function in elderly patients, with simultaneous use of antihypertensive drugs, diuretics or NSAIDs.
Other Precautions
Patients are advised to continue to follow a diet with even carbohydrate intake throughout the day.
Overweight patients are recommended to continue to follow a hypocaloric diet (but not less than 1000 kcal/day). Patients should also exercise regularly.
Patients should tell their doctor about any treatment they are undergoing and any infectious diseases such as respiratory or urinary tract infections.
Standard laboratory tests should be performed regularly to monitor diabetes mellitus.
Metformin does not cause hypoglycemia when used alone, but caution is recommended when used in combination with insulin or other oral hypoglycemic agents (for example, sulfonylureas or repaglinide). Symptoms of hypoglycemia include weakness, headache, dizziness, increased sweating, rapid heartbeat, blurred vision, or difficulty concentrating.
It is necessary to warn the patient that the inactive components of the drug Glucophage® Long can be excreted unchanged through the intestines, which does not affect the therapeutic activity of the drug.
Impact on the ability to drive vehicles and operate machinery
Monotherapy with Glucophage Long does not cause hypoglycemia and therefore does not affect the ability to drive a car or operate machinery.
However, patients should be warned about the risk of hypoglycemia when using metformin in combination with other hypoglycemic drugs (sulfonylureas, insulin, repaglinide).
Overdose Symptoms: when using metformin at a dose of 85 g (42.5 times the maximum daily dose), the development of hypoglycemia was not observed, but in this case the development of lactic acidosis was observed. Significant overdose or associated risk factors can lead to the development of lactic acidosis.
Treatment: if signs of lactic acidosis appear, treatment with the drug must be stopped immediately, the patient must be urgently hospitalized and, after determining the lactate concentration, the diagnosis must be clarified. The most effective measure for removing lactate and metformin from the body is hemodialysis. Symptomatic treatment is also carried out.
Drug interactions Contraindicated combinations
Against the background of functional renal failure in patients with diabetes, radiological examination using iodine-containing radiocontrast agents can cause the development of lactic acidosis. Glucophage Long should be discontinued 48 hours before and not resumed until 48 hours after an X-ray examination using iodinated contrast agents, provided that during the examination renal function was found to be normal.
Combinations not recommended
Ethanol intake increases the risk of developing lactic acidosis during acute alcohol intoxication, especially in the case of malnutrition, low-calorie diet, and liver failure. During treatment, you should not use medications containing ethanol.
Combinations requiring caution
Medicines with indirect hyperglycemic effects (for example, corticosteroids and tetracosactide for systemic and local use), beta2-agonists, danazol, chlorpromazine when taken in high doses (100 mg / day) and diuretics: more frequent monitoring of blood glucose concentrations may be required. especially at the beginning of treatment. If necessary, the dose of Glucophage Long can be adjusted during treatment and after its cessation, based on the level of glycemia.
Concomitant use of loop diuretics may lead to the development of lactic acidosis due to possible functional renal failure. Glucophage Long should not be prescribed if CC is less than 60 ml/min.
Antihypertensive drugs of the ACE inhibitor class can reduce blood glucose concentrations. If necessary, the dose of metformin should be adjusted.
When using the drug Glucophage® Long simultaneously with sulfonylurea derivatives, insulin, acarbose, and salicylates, hypoglycemia may develop.
Nifedipine increases the absorption and Cmax of metformin.
Cationic drugs (amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim and vancomycin) secreted in the renal tubules compete with metformin for tubular transport systems and may lead to an increase in its Cmax.
Conditions for dispensing from pharmacies The drug is dispensed with a prescription. Conditions and periods of storage The drug should be stored out of the reach of children at a temperature not exceeding 25°C. Shelf life: 3 years.
Analogs
Level 4 ATX code matches:
Bagomet
Diaformin
Metformin
Formetin
Gliformin
Glucophage
Siofor
The main analogues of this drug:
- Siofor
- Glucophage Long
- Metformin
- Gliformin
- Merifatin
- Formetin
- Diasfor
- Metadiene
- Diaformin
- Rinformin
Reviews about Glucophage
Quite often, patients leave reviews about Glucophage Long 750 mg, since this is the dosage prescribed for the treatment of type 2 diabetes in its middle stage. However, most patients note sufficient effectiveness of the drug. There are often reports that when diabetics with high body weight took this medicine, they subsequently noticed a moderate reduction in weight to more acceptable levels.
As for Glucophage XR 500, the medicine in this dosage can be prescribed at the initial stage of treatment. In the future, a gradual increase in the dose is allowed until the most effective dose is selected.
It should be noted that any hypoglycemic drugs can only be prescribed by a specialist. In addition to competent drug treatment, the doctor will recommend changes in diet and exercise, which should be an integral part of the life of people suffering from diabetes. Only this approach will ensure a normal quality of life and not so acutely feel all the unwanted symptoms of this disorder.
Glucophage Long is an effective and safe hypoglycemic drug for long-term use
Diabetes mellitus type 2 (DM 2) is a chronic disease characterized by the development of micro- and macrovascular complications. Their prevention is an important task of modern medicine. The article discusses the key links in the pathogenesis of type 2 diabetes, pathophysiologically based approaches to its treatment, in particular the use of drugs whose action is aimed at improving tissue sensitivity to insulin. The pharmacological characteristics of metformin and the possibility of using the drug in patients with chronic kidney disease and non-alcoholic fatty liver disease are discussed in detail. The main advantages of Glucophage Long are reflected.
Table 1. Relative risk of the need to intensify glucose-lowering therapy depending on the starting drug
Rice. 1. Prevalence of CKD among patients with type 2 diabetes
Rice. 2. CKD and the risk of developing hypoglycemic conditions (blood glucose < 2.7 mmol/l)
Table 2. Stages of CKD depending on GFR
Table 3. Indexation of CKD by albuminuria level
Rice. 3. Structure of Glucophage Long tablet
Rice. 4. Frequency of gastrointestinal side effects in patients taking metformin and Glucophage Long since the diagnosis of diabetes
Rice. 5. Frequency of gastrointestinal side effects in patients taking metformin and switched to Glucophage Long
Diabetes mellitus type 2 (DM2) is a dangerous, progressive and very common disease. According to the International Diabetes Federation, in 2014 the number of patients in the world reached 387 million, with type 2 diabetes accounting for 90% of cases [1]. The leading reasons for this unfavorable trend are overweight/obesity, physical inactivity and population aging.
Type 2 diabetes is considered a multifactorial disease associated with various concomitant metabolic disorders. It worsens not only the cardiovascular, but also the overall prognosis [2–5]. The main cause of death in patients with type 2 diabetes is cardiovascular disease (CVD).
According to the World Health Organization, the disease increases overall mortality by two to three times [6].
Chronic complications of type 2 diabetes remain the main problem for most patients. Hyperglycemia is a major risk factor for microvascular complications [7]. Macrovascular complications, as a rule, are caused by other significant factors: visceral obesity, insulin resistance (IR), arterial hypertension, dyslipidemia. In combination with hyperglycemia, they significantly worsen the cardiovascular prognosis and require timely intervention [7–10]. Hypoglycemia increases the risk of developing pathology of the heart and blood vessels, which promotes the activation of contrainsular protection [11–13]. Hypoglycemia is known to be an independent risk factor for cardiovascular events. That is why, when choosing a hypoglycemic drug, factors that increase the risk of its development should be taken into account: age, kidney and liver pathology [14–16].
Excess body weight of patients, which often increases during therapy, especially when taking sulfonylureas (SUMs) can also hinder the effective treatment of diabetes [1, 8, 11].
The progress achieved in recent years in the field of diabetology is largely due to the introduction into clinical practice of modern algorithms for the management of patients with diabetes. When choosing a therapeutic agent, not only its effectiveness but also its safety are taken into account [1, 15, 17].
According to the Russian algorithm for specialized medical care for patients with type 2 diabetes, the start and intensification of glucose-lowering therapy is carried out depending on the initial level of glycated hemoglobin (HbA1c). It is recommended to maintain HbA1c levels within selected individual values depending on age, life expectancy, complications and risk of severe hypoglycemia. If pharmacotherapy is insufficiently effective at each stage, it is necessary to change pharmacotherapy no later than six months from the start of treatment [1].
Metformin is recommended as a first-line drug, given its effectiveness in lowering glycemic levels, lack of effect on body weight, low risk of hypoglycemia, good tolerability and relatively low cost [1, 4, 17].
To date, metformin remains the most studied monotherapy drug [18–21]. Metformin is equally effective and safe in both young and elderly patients [13, 18, 20].
In addition, the choice of insulin resistance (IR), a fundamental pathophysiological mechanism for the development of type 2 diabetes, as a therapeutic target made it possible to improve the sensitivity of organs and tissues to insulin. The results of studies have demonstrated the significant role of IR in the development and progression of CVD, as well as in increasing the risk of acute macrovascular complications [3, 4, 22] and unfavorable prognosis [18, 23]. The degree of IR is an independent predictor of kidney disease progression [24].
Metformin has a pronounced inhibitory effect on IR. It should be noted that the elimination of glucose toxicity due to effective reduction of glucose levels also improves tissue sensitivity to insulin [1, 25, 26].
The UKPDS study also noted the ability of metformin to prevent the development of macrovascular complications. Treatment with metformin, compared with PSM and insulin, improved the prognosis of patients to a greater extent: the risk of death from any cause, death from diabetes or heart attack decreased by 36% [21], which was confirmed in subsequent studies [18, 19].
Effects of metformin
The antihyperglycemic effect of metformin is the result of its effect on insulin sensitivity mainly at the level of the liver, as well as muscle and adipose tissue [25–27]. Metformin reduces glucose production mainly due to the suppression of gluconeogenesis: the expression of the gene that induces this process by phosphorylating cyclic adenosine monophosphate (cAMP), a co-activator of CREB protein, is reduced [27, 28]. In addition, the supply of gluconeogenesis substrates to hepatocytes is reduced and enzymes such as pyruvate carboxylase, fructose-1,6-biphosphatase and glucose-6-phosphatase are inhibited.
It is known that excess production of glucose by the liver at night in patients with type 2 diabetes is especially unfavorable due to the stimulation of atherogenesis and the development of resistance to glucose-lowering drugs. Thus, with an increase in fasting blood glucose (FBG) concentration > 6.1 mmol/l, the risk of developing cardiovascular events in the next 12.4 years increases by 1.33 times [29]. Taking metformin helps reduce the level of GKN by 25–30% (on average by 3.3–3.9 mmol/l) [25, 26].
Under the influence of metformin, tissue sensitivity to insulin increases by 18–50%, resulting in increased utilization of glucose by the liver, muscle and fat tissues. In these tissues, metformin promotes the binding of insulin to receptors. There is also an increase in their quantity and affinity, activation of post-receptor mechanisms of insulin action, in particular tyrosine kinase and phosphotyrosine phosphatase [25, 26].
Treatment with metformin also changes the lipid profile: the concentration of triglycerides decreases by 10–20%, low-density lipoprotein cholesterol (LDL) cholesterol by 10%, while the concentration of high-density lipoprotein cholesterol (HDL) increases by 10–20% [20, 25]. Metformin helps reduce the level and rate of oxidation of free fatty acids (by 10–17 and 10–30%, respectively) and activate their re-esterification. As a result, the effects of lipotoxicity are eliminated at all levels, including the liver, adipose and muscle tissue, and the islets of Langerhans [26].
The intestinal effect of metformin is to slow down the rate of absorption of carbohydrates. At the same time, the drug increases the utilization of glucose in the gastrointestinal tract (GIT), enhancing anaerobic glycolysis both in a state of saturation and on an empty stomach. As a result, postprandial glycemia decreases by an average of 20–45% [20, 25]. Thus, metformin makes a significant contribution to the prevention of postprandial peaks in glycemia associated with the risk of premature death from CVD [20].
Prevention of hypoglycemia, given its dangerous consequences, in patients with type 2 diabetes with CVD is extremely important [4, 6, 10]. Thanks to these effects of metformin, glucose levels decrease without the risk of hypoglycemic conditions, which is an undoubted advantage of the drug [20]. The use of metformin leads to a decrease in HbA1c levels by 1.5–2.0% [1, 25].
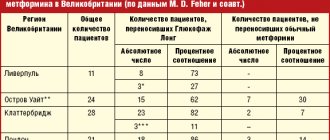
It is important to note that, without having direct effects on pancreatic beta cells, metformin improves insulin secretion, helping to maintain their functional activity. As IR decreases, the basal level of insulin in the blood serum decreases [20, 25]. In this regard, the results of a retrospective study [17], which analyzed hypoglycemic therapy at the initiation and intensification stages, are interesting. The number of participants was 15,516. The observation period was from 2009 to 2013. Depending on the treatment received, patients were divided into groups: metformin, PSM, thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors. The purpose of the study was to determine the initiation of intensification of therapy (adding another antihyperglycemic agent, including insulin) in patients with type 2 diabetes receiving oral antihyperglycemic drugs (OLDs) for the first time.
Only 57.8% of patients started therapy with metformin. Using Cox regression analysis, it was found that starting therapy with metformin (compared with other DSPs) was associated with a lower need for intensifying therapy in the future (p
Considering that the vast majority of patients with type 2 diabetes are overweight, the primary goal of treatment is to reduce it and maintain it at normal levels [1, 9]. During therapy with metformin, patients experience a decrease in body weight or no increase in weight. In addition, treatment is accompanied by a decrease in visceral fat deposition, which is an independent risk factor for the development of CVD [20, 25]. Recent studies indicate that metformin suppresses the production of the orexigenic peptide ghrelin and increases the level of glucagon-like peptide 1, which has an anorexigenic effect. This partly explains some of the drug's metabolic effects [20, 30].
In recent years, the cardioprotective effects of metformin have been actively discussed [31]. By suppressing increased adhesion of monocytes to the vascular endothelium and lipoidosis, metformin affects the mechanisms of development of atherosclerosis [32, 33]. The drug accelerates the catabolism of LDL, promoting their conversion to HDL, reduces the accumulation of cholesterol esters in the aorta, increases the content of phospholipids and reduces the content of sphingomyelin. Along with this, metformin reduces the proliferation of vascular smooth muscle cells, suppresses the processes of differentiation of monocytes into macrophages, which actively secrete proatherogenic factors. In vitro, metformin inhibited leukocyte-endothelial interaction, as well as endothelial surface expression of intracellular adhesion molecule 1, vascular cell adhesion molecule 1 and E-selectin [25, 26]. The drug has been shown to have a positive effect on the hemostatic system, blood rheology, endothelial function and vascular reactivity [34, 35].
The results of a number of studies have made it possible to reveal other mechanisms underlying the cardioprotective effect of the drug. Thus, in studies by K. Isoda et al. Metformin has been demonstrated to dose-dependently inhibit the release of interleukins (IL) 6 and 8 induced by IL-1-beta in vascular smooth muscle cells, macrophages and endothelial cells [36]. The authors suggest that these processes are based on a decrease in NF-kB translocation.
Since the clinical significance of these properties of metformin has not been conclusively confirmed, their further study is of interest.
Use of metformin in CKD
Diabetic nephropathy is one of the most serious and disabling complications of type 2 diabetes. The risk of developing chronic kidney disease (CKD) in diabetes increases 2.6 times [37, 38]. The disease is detected in approximately one third of patients (Fig. 1).
Diabetic nephropathy is in second place among the causes of death after CVD. It is the main cause of the development of end-stage CKD. In terms of the need for hemodialysis and kidney transplantation, patients with diabetes still hold the lead [14, 16, 39].
Impaired renal function limits the choice of hypoglycemic agent [1, 16, 39] due to the increased risk of hypoglycemia (Fig. 2) due to reduced creatinine clearance, as well as impaired renal gluconeogenesis [2, 16, 39].
The concept of CKD was introduced to unify approaches to the diagnosis, treatment and prevention of kidney damage. It combines various kidney damage and/or decreased function that persists for three months or more, regardless of the primary diagnosis [1, 2]. To make a diagnosis of CKD in the case of preserved or increased glomerular filtration rate (GFR), as well as its slight decrease (60 ≤ GFR
- albuminuria ≥ 30 mg/day or urine albumin/creatinine (al/cr) ratio ≥ 30 mg/g (≥ 3 mg/mol);
- change in urine sediment;
- electrolyte disturbances;
- structural and morphological changes;
- history of kidney transplantation.
With GFR
Assessment of renal dysfunction is necessary not only for the primary diagnosis of kidney pathology, but also for monitoring the effectiveness and safety of therapy, the rate of progression of the pathological process and determining the prognosis. The stage of renal dysfunction is determined by the GFR value, as it most fully reflects the number and total volume of nephron work (Table 2), taking into account the level of albuminuria (Table 3).
The basis for introducing the classification of CKD according to the level of albuminuria was the data that the risk of overall and cardiovascular death and progression of CKD in any range of GFR depends on the rate of albumin excretion (AER) in urine [31].
Of course, the adverse consequences of CKD can be prevented or delayed if diagnosed and treated early [14, 16, 40].
The possibility of using metformin in CKD is currently being actively discussed [40, 41]. It should be noted that metformin is not metabolized in the body and is excreted primarily by the kidneys. With GFR
To prevent lactic acidosis, before prescribing the drug, it is necessary to carefully examine patients in order to identify contraindications to its use. Contraindications include:
- diseases accompanied by tissue hypoxia (heart or pulmonary failure, myocardial infarction, anemia, etc.);
- renal failure or impaired renal function (creatinine clearance
- liver failure, alcoholism;
- pregnancy, lactation;
- acute conditions that may impair renal function (dehydration, acute infection, shock, intravascular administration of radiocontrast agents);
- diabetic ketoacidosis.
Retrospective evaluation of metformin use in University of Chicago patients from 2004–2009. and patients participating in the NHANES study, 1999–2006. demonstrated that metformin use is quite common in GFR
The use of metformin at a GFR of 45–50 ml/min/1.73 m² is safe in the absence of other risk factors for the development of lactic acidosis: poorly controlled diabetes, ketoacidosis, prolonged fasting, excessive alcohol consumption, liver failure and conditions associated with severe hypoxia [1, 42 ].
Use of metformin in NAFLD
In patients with type 2 diabetes, gastrointestinal diseases are often observed, among which non-alcoholic fatty liver disease (NAFLD) is the leading one. The concept of NAFLD combines clinical and morphological changes in the liver - from fatty hepatosis, non-alcoholic steatohepatitis to fibrosis with a possible outcome in the form of cirrhosis, developing due to the influence of various factors in patients who do not drink alcohol in hepatotoxic doses [44, 45]. Since the development of NAFLD is associated with IR, the former is diagnosed in 50–78% of patients with type 2 diabetes. NAFLD, in turn, contributes to the development of CVD [44, 45]. Therefore, the use of metformin in the combination of type 2 diabetes and NAFLD is pathogenetically justified.
As already noted, the main mechanism of action of metformin is realized through the activation of cAMP-dependent protein kinase of the liver, which is accompanied by a decrease in the synthesis of triglycerides from fatty acids and suppression of mitochondrial beta-oxidation, a decrease in the expression of tumor necrosis factor alpha and transcription factors responsible for the synthesis of cholesterol from acetyl-coenzyme A [20, 25, 28].
The results of a retrospective study (2000–2010) including patients with type 2 diabetes with cirrhosis (n = 250) showed that patients receiving metformin (n = 172) at the time of diagnosis of cirrhosis, compared with patients ( n = 78), for whom metformin was discontinued at this stage, the five-year survival rate statistically significantly increased (11.8 versus 5.6 years, p
Glucophage Long
It has been established that 5–10% of patients with diabetes stop taking metformin due to negative events from the gastrointestinal tract [20, 47]. Metformin sustained release, the drug Glucophage Long, can increase the effectiveness of therapy, reduce the frequency of adverse reactions and, as a result, increase patient adherence to treatment [47, 48]. This dosage form appeared thanks to the creation of a tablet with a diffusion system through a gel barrier (Fig. 3). The active substance is contained within a two-layer hydrophilic polymer matrix (inner polymer matrix), surrounded by a closed polymer matrix (outer polymer matrix). After taking the drug, the polymers of the outer dense layer are hydrated and the Glucophage Long tablet turns into a gel-like mass, increasing in size. This transformation helps slow down evacuation through the pylorus and increases the time the drug remains in the stomach. The drug, released for absorption from the inner layer, diffuses through the outer polymer matrix. The release of 90% of the contained drug substance takes about 10 hours, in contrast to the traditional form, when 90% of metformin is released within 30 minutes.
It is important to note that the rate of release of the substance does not depend on intestinal motility or pH level, which minimizes the variability of drug delivery to the gastrointestinal tract.
Pharmacokinetic studies have shown that after a single dose of 2000 mg metformin sustained release, the area under the concentration-time curve was similar to that after a double dose of 1000 mg metformin regular release, indicating the bioequivalence of these dosage forms [48, 49].
It has been proven that the time to reach peak concentration of Glucophage Long increases to 7 hours (for regular release metformin it is 2.5 hours) [49]. Consequently, Glucophage Long has a longer action, which allows it to be taken once a day. This in turn helps to improve adherence and treatment outcomes for type 2 diabetes [47–49].
With similar bioavailability, the peak concentration of sustained-release metformin is reduced by 25% compared to that of regular-release metformin [49].
In a randomized, double-blind study, Glucophage Long demonstrated the same effectiveness in lowering HbA1c levels as regular-release metformin [50].
In addition, thanks to the pharmacokinetics of Glucophage Long, it is possible to avoid a rapid increase in plasma metformin concentrations and, as a consequence, the development of adverse events from the gastrointestinal tract (Fig. 4) [47–50]. Thus, a retrospective analysis of medical records of patients with type 2 diabetes for gastrointestinal tolerability of two forms of metformin showed a significant reduction in the incidence of adverse events from the gastrointestinal tract in patients transferred from therapy with regular-release metformin to sustained-release metformin (Fig. 5) [47, 48] .
Glucophage Long is available in tablets of 500 and 750 mg. The initial dose is 500 mg once a day. The drug is taken during dinner. Its dose, depending on the level of glucose in the blood plasma, can be increased by 500 mg every 10–15 days to a maximum daily dose of four 500 mg tablets or three 750 mg tablets once. If the target glycemic level is not achieved at the maximum daily dose, the possibility of taking the drug twice a day is considered. When switching from Glucophage to Glucophage Long, the initial dose of the latter should be equal to the daily dose of the former.
Conclusion
The choice of PSP requires a balanced approach and assessment of the risk/benefit ratio, especially in patients with risk factors for CVD and CKD. The effectiveness of glucose-lowering therapy can be increased by the use of drugs that affect IR. Metformin has a pronounced inhibitory effect on IR. To date, it remains the most studied drug in terms of effectiveness and safety in the treatment of patients with type 2 diabetes, both in monotherapy and in combination with other PSPs and insulin. Modern prolonged forms of metformin (Glucophage Long) retain all the advantages of traditional metformin, and are also characterized by better tolerability and ease of use, which helps to increase treatment adherence.
Price Glucophage Long, where to buy
The price of Glucophage Long 500 mg for 30 pieces per package is about 200 rubles.
- Online pharmacies in RussiaRussia
ZdravCity
- Glucophage Long tablets with prolong release.
750 mg 60 pcs. LLC Nanolek 467 rub. order - Glucophage long tablets prolong. 750 mg 30 pcs. Nanolek LLC
RUB 286 order
- Glucophage long tablets prolong. 500 mg 60 pcs. Merck Sante S.A.S./Nanolek LLC
RUB 332 order
- Glucophage Long tab. with prolong. release 1000 mg No. 60 LLC Nanolek
RUR 641 order
- Glucophage Long tablets prolonged action 500 mg 30 pcs. Nanolek LLC
170 rub. order