Place of ropinirole in initial therapy for Parkinson's disease
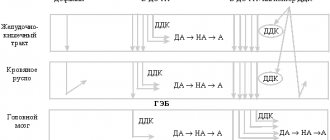
Krasnov M.Yu. Parkinson's disease (PD) is the second most common neurodegenerative disease after Alzheimer's disease [1]. There are from 100 to 250 cases of the disease per 100 thousand population [21], and the incidence is directly proportional to age. Thus, the average age of onset of the disease is estimated at 58-60 years [19], however, recent data on the epidemiology of parkinsonism challenge the idea of its “age-dependent” nature: approximately every tenth patient becomes ill before the age of 50, and every twentieth - before the age of 40. years [1]. Considering that the average retirement age in economically developed countries is 65 years, it is clear that the burden of the disease is clearly socio-economic in nature. Due to the large number of working and socially active patients, the first place in the treatment of the disease comes to the development of drugs that can not only slow down the development of parkinsonism, but also maintain the quality of life of patients at a high enough level to maintain their working capacity. Many years of studying methods of drug therapy for Parkinson's disease have led to the fact that a practical neurologist has in his arsenal drugs designed to treat the early (uncomplicated) stage of the disease. The main advantages of this constantly improving group of drugs are a good tolerability profile and the existence of long-acting forms designed for once daily use, which increases patient adherence to treatment. In the monotherapy of Parkinson's disease, the leading place is occupied by dopamine receptor agonists (DRAs), which have convincingly proven their effectiveness in the treatment of both motor and non-motor symptoms over many years of use in clinical practice. Currently, the pharmacological market includes 10 drugs from the ADR spectrum, 5 of which are derivatives of ergot alkaloids (older generation drugs), while the rest (apomorphine, piribedil, pramipexole, rotigotine and ropinirole) are derivatives of other compounds. In this series, a prominent place is occupied by ropinirole, registered in 1991 and over the entire period of observation, which has repeatedly confirmed its safety and therapeutic effectiveness. Its symptomatic antiparkinsonian activity is due to D2/D3 mimetic effects; Due to the presence of additional (including antioxidant and antiapoptotic) properties, ropinirole, along with other ADRs, can also be considered as a potential neuroprotector [4, 16]. The neuroprotective effects of ADR and ropinirole, in particular, can be realized through presynaptic dopamine D2 receptors that regulate dopamine synthesis, as well as through inhibition of glutamate neurotoxicity associated with excessive activity of the subthalamic nucleus [13].
Controlled-release ropinirole is as effective as immediate-release ropinirole [14], and clinical studies have demonstrated the ability to safely switch between formulations while maintaining an equivalent dose. There is class I evidence that early use of ropinirole in PD is accompanied by a lower likelihood of developing motor complications compared to levodopa [10, 18]; Also, when using ropinirole, it was proven that the risk of motor complications remains lower, provided that treatment was started with monotherapy [6].
Ropinirole also has the ability to reduce depression in PD [11]. Nocturnal sleep disturbances assessed using the PD Sleep Scale (PDSS) have demonstrated improvements in most measures of sleep quality in patients taking ropinirole [9]. Improving the quality of sleep is primarily achieved due to the effect of ropinirole on the “night” symptoms of PD, both motor and non-motor: increased manifestations of parkinsonism at night and early morning hours, restless leg syndrome, pain, obsessive and nightmare dreams, nocturia, etc. P. In addition to improving the quality of sleep, taking ropinirole can also significantly reduce the severity of motor manifestations upon awakening, which ensures optimal motor activity for the patient in the first half of the day (which is important for younger and working patients). Symptomatic control of parkinsonism has been demonstrated for both controlled- and immediate-release ropinirole (recommendation level A); data on this parameter for ropinirole are more reliable than for amantadine and anticholinergic drugs [4]. Ropinirole remains effective when prescribed in late, complicated stages of PD: its ability to reduce the duration of the “off” period in patients with motor fluctuations is known; this is true for both immediate [8, 10] and controlled release [9] forms. The decrease in the “off” period when ropinirole is added to levodopa is 1.17 hours [15]; the effective dose in this case ranges from 8 to 16 mg [20].
The use of a modified dosage form of ropinirole (controlled release) allows for higher plasma concentrations of the drug, more constant dopaminergic stimulation of receptors and improved tolerability of the drug [2, 3]. The effectiveness of ropinirole with controlled (prolonged) action and the comparability of equal doses of both forms of the drug were previously confirmed in clinical studies, while the degree of adherence to treatment was significantly higher in patients taking long-acting ropinirole (97% versus 88%) [3, 6] . The good efficacy and tolerability of ropinirole make it possible to ensure not only optimal control of the manifestations of PD, but also to delay the administration of levodopa or limit it to its minimum dose, which, in turn, prospectively reduces the risk of motor fluctuations and drug-induced dyskinesias [2]. In a five-year comparative study of the use of ropinirolol and levodopa, the incidence of dyskinesias was 20% for ropinirole, while for levodopa it was 45% [10]. The possibility of taking the drug once a day and a flexible dose titration scheme provide additional opportunities to achieve high adherence to treatment, which ultimately also increases the effectiveness of the therapy [17]. The good tolerability profile of ropinirole usually allows the optimal therapeutic dose to be achieved in a relatively short time.
The availability of various dosage forms of ropinirole (2, 4 and 8 mg, including controlled release) opens up wide opportunities for a highly individual, personalized approach to the treatment of PD in each patient. It is advisable to start treatment with a dose of 2 mg once a day and, subject to good tolerance, increase the dose every 7 days until the individual daily requirement is reached (on average - 8-12 mg/day, maximum - up to 24 mg/day) [2]. The safety of prescribing ropinirole and the low incidence of side effects, provided that a competent approach to dose escalation is followed, remains comparable both when ropinirole is prescribed as initial therapy and when it replaces other ADRs (simultaneously or using a slow transition scheme from one drug to another). This transition is usually carried out taking into account empirically selected equivalent doses: 1 mg pramipexole = 10 mg bromocriptine = 100 mg piribedil = 4 mg ropinirole [2, 5]. However, it should be remembered that if any ADR is replaced by ropinirole due to poor tolerability of the original drug, then the drug should be given at a dose lower than the equivalent one, followed by titration to the required one. The advantages of ropinirole over other ADRs were shown in a meta-analysis of 23 studies of the effectiveness of monotherapy with non-ergoline ADRs: by the 24-28th week of therapy, the total score on parts II and III of the UPDRS scale decreased during treatment with rotigotine by 5.35 points, with pramipexole - by 6.06 , ropinirole - by 6.32 [15].
The side effects of ADRs are related to their effects on peripheral dopamine receptors. The most common side effects of ropinirole are similar to those of other non-ergoline ADRs; these include peripheral edema (most often of the lower extremities), daytime drowsiness, nausea, dizziness, and orthostatic hypotension. The number of patients in whom the severity of the side effects of ropinirole requires its discontinuation does not exceed 5% [7]. As a rule, most side effects in patients taking ADRs are either temporary and go away on their own within 2-4 weeks from the start of treatment, or are dose-dependent and can be easily adjusted by optimizing the dose or selecting concomitant therapy (for example, temporary use is possible domperidone for gastrointestinal motility disorders at the initial stage of ropinirole therapy [4]; the use of fludrocortisone as a corrector of orthostatic hypotension [12]).
To summarize: ropinirole meets all the requirements of a drug that forms maximum adherence to treatment: the possibility of a single dose and a wide range of effective doses, together with a good tolerability profile and a low frequency of side effects, make it the drug of choice both among ADRs and among the range of other antiparkinsonian drugs. Convenience of administration and low likelihood of long-term adverse events (especially in comparison with levodopa) make it reasonable to consider the prescription of ropinirole in the initial stages of PD, both as monotherapy and in combination with other drugs. The possibility of long-term maintenance of optimal quality of life and maintaining high social activity against the background of adequate antiparkinsonian therapy also makes the clinical use of ropinirole economically feasible as a drug that allows the formation of a flexible strategy for the treatment of PD throughout the entire course of the disease.
REFERENCES 1. Illarioshkin S.N., Ivanova-Smolenskaya I.A. Trembling hyperkinesis: A guide for doctors. - M., 2011. - P. 65-6.
2. Levin O.S., Datieva V.K. Main aspects of the use of extended-release ropinirole (requip modutab) in the treatment of Parkinson's disease // Neurology, neuropsychiatry, psychosomatics. - 2013. - No. 1. — P. 46-9.
3. Antonini A., Tolosa E., Mizuno Y. A reassessment of risks and benefits of dopamine agonists in Parkinson's disease // Lancet Neurol. - 2009. - Vol. 8, No. 10. - P. 929-37.
4. Ferreira JJ, Katzenschlager R, Bloem BR et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson's disease // Eur J Neurol. — 2013. — Vol. 20, no. 1. — P. 5-15.
5. Grosset DG, Grosset KA, Okun MS, Fernandez HH Parkinson's disease // Manson Pub Ltd. - 2009. - P. 63-6.
6. Hauser RA, Rascol O., Korczyn AD et al. Ten–year follow–up of Parkinson’s disease patients randomized to initial therapy with ropinirole or levodopa // Mov Disord. - 2007. - Vol. 22. - P. 2409–17.
7. Hersh BP Earl NL, Hauser RA, Stacy M. Early treatment benefits of ropinirole prolonged release in Parkinson's disease patients with motor fluctuations // Mov Disord. - 2010. - Vol. 25, No. 7. - P. 927-31.
8. Lieberman A., Olanow C.W., Sethi K. et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group // Neurology. - 1998. - Vol. 51. - P. 1057–62.
9. Pahwa R, Stacy MA, Factor SA et al. Ropinirole 24–hour extended release: randomized, controlled study in advanced Parkinson disease // Neurology. - 2007. - Vol. 68. - P. 1108–15.
10. Rascol O., Brooks D. J., Korczyn AD et al. A five–year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa // N Engl J Med. - 2000. - No. 342. - P. - 1484–91.
11. Rektorova I., Balaz M., Svatova J. et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study // Clin Neuropharmacol. - 2008. - Vol. 31, No. 5. - P. 261-6.
12. Schoffer KL,. Henderson R.D. O'Maley K., O'Sullivan JD Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson's disease // Mov Disord. - 2007. - Vol. 22, No. 11. - P. 1543-9.
13. Stocchi F. Neuroprotection in Parkinson's disease: clinical trials // Parkinson's disease and related disorders. - 2007. - P. 17-9.
14. Stocchi F, Hersh BP, Scott BL et al. The Ease–PD Monotherapy Study Investigators. Ropinirole 24–hour extended release and ropinirole immediate release in early Parkinson's disease: a randomized, double–blind, non–inferiority crossover study // Curr Med Res Opin. - 2008. - Vol. 24. - P. 2883–95.
15. Thorlund K., Wu P. Druyts E. et al. Nonergot dopamine-receptor agonists for treating Parkinson's disease — a network meta-analysis // Neuropsychiatr Dis Treat. — 2014. — Vol. 10. - P. 767-76.
16. Tolosa E., Marin C. Dopamine agonists in Parkinson's disease: a clinical review // In: Beyond the Decade of Brain. Dopamine agonists in early Parkinson's disease. - 1997. - Vol. 12. - P. 143-61.
17. Watts RL, Lyons KE, Pahwa R. et al. Onset of dyskinesia with adjunct ropinirole pro-longed-release or additional levodopa in early Parkinson's disease // Mov Dis. - 2010. - Vol. 25. - P. 858-66.
18. Whone AL, Watts RL, Stoessl AJ et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study // Ann Neurol. - 2003. - Vol. 54, no. 1. - P. 93-101.
19. Wolters EC, Bosboom JLW Parkinson's Disease // In: Parkinsonism and Related Disorders. — Amsterdam, VU University Press. - 2007. - P. 154.
20. Zesiewicz T.A., Chriscoe S., Jimenez T. et al. A randomized, fixed-dose, dose-response study of ropinirole prolonged release in advanced Parkinson's disease // Neurodegener Dis Manag. — 2021. — Vol. 7, No. 1. — P. 61-72.
21. Zhang ZX, Roman GC, Hong Z. et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai // Lancet. - 2005. - Vol. 365. - P. 595-7.
Attending doctor…
REQUIP MODUTAB
Directions for use and doses
Adults
Inside.
Requip Modutab® should be taken once a day at the same time, regardless of meals. Take the tablets whole, without chewing or breaking.
The need for dose titration should be considered if one or more doses are missed.
A dose reduction is recommended if the patient experiences drowsiness at any stage of dose selection.
If other adverse reactions develop, it is necessary to reduce the dose of the drug followed by a gradual increase in dose.
It is recommended to individually select the dose in accordance with the effectiveness and tolerability of the drug.
Monotherapy
Start of treatment
The recommended starting dose of Requip Modutab® is 2 mg once a day for one week. Subsequently, the dose is increased by 2 mg at intervals of at least 1 week to 8 mg/day.
| A week | 1 | 2 | 3 | 4 |
| Daily dose (mg) | 2 | 4 | 6 | 8 |
Maintenance dose
If, after selecting the dose, the therapeutic effect is not sufficiently pronounced or is unstable, you can continue to increase the daily dose of the drug by 4 mg at intervals of 1-2 weeks (until the required therapeutic effect is achieved). The dose may be adjusted depending on the therapeutic effect and increased to a maximum dose of 24 mg once daily.
Combination therapy
When using Requip Modutab® in doses used for monotherapy in combination with levodopa drugs, the dose of levodopa can be gradually reduced (depending on the clinical effect). In clinical studies in patients concomitantly receiving Requip Modutab® extended-release tablets, the dose of levodopa was gradually reduced by approximately 30%. In patients with a progressive form of the disease taking Requip Modutab® in combination with levodopa drugs, dyskinesia may occur during the dose titration period of ropinirole. Reducing the dose of levodopa may reduce these symptoms.
Cancellation of therapy
As with other dopaminergic drugs, Requip Modutab® should be discontinued by gradually reducing the daily dose over at least 1 week. If treatment was interrupted for 1 day or longer, the need for dose titration should be considered when resuming therapy.
Special patient groups
Elderly patients
Despite a possible decrease in drug clearance in patients aged 65 years and older, dose titration of ropinirole in this category of patients is carried out as usual.
Patients with impaired renal function
Mild to moderate renal impairment
In patients with mild to moderate renal impairment (creatinine clearance 30-50 ml/min), the clearance of ropinirole does not change, and no dose adjustment of ropinirole is required.
Patients with end-stage renal disease undergoing hemodialysis
The recommended starting dose of ropinirole is 2 mg once daily. Subsequent dose increases should be based on assessment of tolerability and effectiveness. The maximum daily dose in patients on continuous hemodialysis is 18 mg. Administration of maintenance doses after hemodialysis is not required.