Phenylephrine-Optik Eye drops 2.5% dropper bottle, 5 ml
Registration Certificate Holder
LECCO (Russia)
Dosage form
Medicine - Phenylephrine-optic
Description
Eye drops
in the form of a transparent, colorless or brownish-yellow liquid.
1 ml
phenylephrine hydrochloride 25 mg
Excipients
: benzalkonium chloride (in terms of anhydrous substance) - 0.1 mg, disodium edetate - 1 mg, sodium hydroxide - 0.24 mg, sodium disulfite - 3 mg, citric acid monohydrate - 1 mg, sodium citrate dihydrate - 5 mg, purified water - up to 1 ml.
5 ml - polymer dropper bottles (1) - cardboard packs. 10 ml - polymer dropper bottles (1) - cardboard packs.
Indications
Orally and locally: to reduce swelling of the mucous membrane of the nasopharynx and conjunctiva during colds and allergic diseases (mainly as part of combination drugs).
Parenteral: to increase blood pressure during collapse and arterial hypotension due to decreased vascular tone.
Contraindications for use
Arterial hypertension, severe atherosclerosis, tendency to spasms of coronary vessels, increased sensitivity to phenylephrine.
pharmachologic effect
Adrenomimetic. It has a direct stimulating effect mainly on α-adrenergic receptors.
When used systemically, it causes constriction of arterioles, increases peripheral vascular resistance and blood pressure. Cardiac output does not change or decreases, which is associated with reflex bradycardia (increased vagal tone) in response to arterial hypertension. Phenylephrine does not increase blood pressure as sharply as norepinephrine and epinephrine, but it acts longer. This is apparently due to the fact that phenylephrine is more stable and is not destroyed by COMT.
When applied topically, phenylephrine has a pronounced vasoconstrictor effect, causes mydriasis, and can lower intraocular pressure in open-angle glaucoma.
In average therapeutic doses it has virtually no effect on the central nervous system.
Drug interactions
When using phenylephrine against the background of general anesthesia induced by halothane or cyclopropane, ventricular fibrillation may develop.
When used simultaneously with MAO inhibitors, a potentiation of the effects of phenylephrine is observed (including when applied topically).
Phenothiazines, alpha-blockers (phentolamine), furosemide and other diuretics reduce the vasoconstrictor effect of phenylephrine.
Guanethidine enhances the mydriatic effect of phenylephrine (with systemic absorption).
Oxytocin, ergot alkaloids, tricyclic antidepressants, furazolidone, procarbazine, selegiline, sympathomimetics increase the pressor effect, and the latter also increase arrhythmogenicity.
When used simultaneously, beta-blockers reduce pacemaker activity; against the background of reserpine, arterial hypertension is possible (due to depletion of catecholamine reserves in adrenergic neurons, sensitivity to sympathomimetics increases).
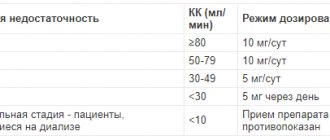
Dosage regimen
When taken orally and topically, the dose depends on the indications and the dosage form used.
When administered subcutaneously or intramuscularly, a single dose is 2-5 mg, then, if necessary, 1-10 mg. When administered intravenously as a stream (slowly), the single dose is 100-500 mcg. With intravenous infusion, the initial rate is 180 mcg/min, then, depending on the effect, it is reduced to 30-60 mcg/min.
Maximum doses:
for adults, when taken orally, a single dose is 30 mg, a daily dose is 150 mg; subcutaneous or intramuscular single dose - 10 mg, daily dose - 50 mg; with intravenous administration, a single dose is 5 mg, daily - 25 mg.
Side effect
From the cardiovascular system:
an undesirable prolonged increase in blood pressure, tachycardia or reflex bradycardia is possible.
Local reactions:
Possibly irritating to mucous membranes.
special instructions
Phenylephrine should be avoided in patients with severe hyperthyroidism.
Use with caution in case of ischemic heart disease.
When applied topically after absorption through the mucous membrane, phenylephrine can cause systemic effects. Therefore, the use of phenylephrine 10% eye drops should be avoided in infants and elderly patients.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated. Restrictions when breastfeeding - Contraindicated.
Adequate and strictly controlled clinical studies of the safety of phenylephrine during pregnancy and lactation have not been conducted.
Use in elderly patients
Restrictions for elderly patients - Contraindicated.
When applied topically after absorption through the mucous membrane, phenylephrine can cause systemic effects. Therefore, the use of phenylephrine 10% eye drops should be avoided in elderly patients.
Use in children
Restrictions for children - Contraindicated.
When applied topically after absorption through the mucous membrane, phenylephrine can cause systemic effects. Therefore, the use of phenylephrine 10% eye drops should be avoided in infants.
Phenylephrine-optic eye drops 2.5% 5 ml x1
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION, INSTRUCTIONS FOR MEDICAL ADMINISTRATION, PHENYLEPHRINE-OPTIC, Registration number: LP-001556 Trade name: Phenylephrine-Optic. International nonproprietary name: phenylephrine. Dosage form: eye drops. Composition per 1 ml. Active ingredient: phenylephrine hydrochloride - 25.0 mg. Excipients: benzalkonium chloride, in terms of anhydrous substance , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , . mg, citric acid monohydrate - 1.0 mg, sodium citrate dihydrate - 5.0 mg, purified water - up to 1 ml. Description: transparent, colorless or brownish-yellow liquid.
Pharmacotherapeutic group: α-adrenergic agonist. ATX code: S01FB01. , Pharmacological properties
Pharmacodynamics Phenylephrine is an α-adrenomimetic, has a pronounced, non-selective, α-adrenomimetic effect. When used in therapeutic doses, it does not have a significant stimulating effect on the central nervous system. When applied topically in ophthalmology, it causes dilation of the pupil, improves the outflow of intraocular fluid and constricts the vessels of the conjunctiva. Weakly affects β-adrenergic receptors, including the heart (does not have a positive chrono- and inotropic effect). The drug has a vasoconstrictor effect similar to the action of norepinephrine (norepinephrine). The vasopressor effect of phenylephrine is weaker than that of norepinephrine, but is longer lasting. Causes vasoconstriction 30-90 seconds after instillation, duration 2-6 hours. After instillation, phenylephrine causes contraction of the dilator pupillary muscle, thereby causing dilation of the pupil. Mydriasis occurs within 10-60 minutes after a single instillation. Mydriasis persists for 2 hours. Mydriasis caused by phenylephrine is not accompanied by cycloplegia.
Pharmacokinetics Phenylephrine easily penetrates into the tissues of the eye; the maximum concentration in plasma is achieved 10-20 minutes after instillation into the eye. Pre-instillation of local anesthetics may increase systemic absorption of phenylephrine and prolong mydriasis. Phenylephrine is excreted in the urine in unchanged form (<.20%) or in the form of inactive metabolites. , Indications for use Iridocyclitis (for, prevention, occurrence of, posterior, synechiae, and, reduction of exudation from the iris). For diagnostic pupil dilation during ophthalmoscopy and other diagnostic procedures necessary to monitor the condition of the posterior segment of the eye. Conducting a provocative test in patients with a narrow anterior chamber angle profile and suspected angle-closure glaucoma. Differential diagnosis of superficial and deep injection of the eyeball. “Red eye” syndrome (for a decrease in hyperemia and irritation of the membranes of the eye). Spasm of accommodation. , Contraindications Hypersensitivity to the components of the drug. Narrow-angle or closed-angle glaucoma. Elderly age. Severe cardiovascular or cerebrovascular diseases. Arterial hypertension in combination with ischemic heart disease, aortic aneurysm, atrioventricular block I-III degree, arrhythmia. Tachycardia. Impaired tear production. Prematurity, children under 6 years of age (with accommodation spasm). Hyperthyroidism. Hepatic porphyria. Congenital deficiency of glucose-6-phosphate dehydrogenase. , Rhinitis. , With caution Diabetes (risk of increased blood pressure associated with impaired autonomic regulation). Concomitant use with monoamine oxidase inhibitors (including within 21 days after stopping their use). Sickle cell anemia, wearing contact lenses after surgery (decreased healing due to conjunctival hypoxia). , , Use during pregnancy and breastfeeding There is no sufficient experience with the use of phenylephrine during pregnancy. Therefore, the use of the drug as prescribed by the attending physician during pregnancy is possible if the potential benefit for the mother exceeds the possible risk for the fetus. It is unknown whether the drug is excreted in breast milk. When prescribing the drug, it is recommended to stop breastfeeding for the duration of treatment. , Method of administration and dosage Locally. For iridocyclitis, the drug is used to prevent the development and rupture of already formed posterior synechiae to reduce exudation into the anterior chamber of the eye. , , , , , , For this purpose, 1 drop of the drug is instilled into the conjunctival sac of the affected eye(s) 2-3 times a day. When performing ophthalmoscopy, a single instillation of the drug is used. As a rule, to create mydriasis, it is enough to instill 1 drop of the drug into the conjunctival sac. Maximum mydriasis is achieved after 15-30 minutes and persists for 1-3 hours. If it is necessary to maintain mydriasis for a long time, re-instillation of the drug is possible after 1 hour. For , diagnostic , procedures , single , instillation , of the drug is used : — , as , a , provocative , test , in , patients , with , a , narrow , profile , of the , angle , of the anterior chamber , of the eye , and , suspected , of the , angle-closure , glaucoma. If the difference between the intraocular pressure values before instillation of the drug and after pupil dilation is from 3 to 5 mm Hg. Art., then, the provocative test is considered positive, for the differential diagnosis of the type of injection of the eyeball: if, 5 minutes after instillation of the drug, constriction of the vessels of the eyeball is noted, then the injection is classified as , superficial, , if , redness , of the eye persists, it is necessary to carefully examine the patient for the presence of iridocyclitis or scleritis, as this indicates dilation of deeper vessels. For, relief, spasm, accommodation, in children, from 6 years old, and in adults, the drug is instilled, 1 drop in each eye at night, every day for 4 weeks. , Side effects From the organ of vision Conjunctivitis, periorbital edema. In some cases, patients note a burning sensation at the beginning of use, blurred vision, irritation, a feeling of discomfort in the eye, increased lacrimation, increased intraocular pressure. Phenylephrine may cause reactive miosis the day after use. Repeated instillations of the drug over a short period of time can lead to less pronounced mydriasis than previously observed. This effect is more likely to occur in older patients. Due to a significant contraction of the muscle that dilates the pupil, 30-45 minutes after instillation under the influence of phenylephrine, pigment particles from the pigment layer of the iris may be detected in the moisture of the anterior chamber of the eye. Suspension in the chamber fluid must be differentiated from manifestations of anterior uveitis or from the ingress of blood cells into the fluid of the anterior chamber. Systemic reactions From the skin and its appendages: contact dermatitis. From the cardiovascular system: palpitations, tachycardia, arrhythmia, increased blood pressure, ventricular arrhythmia, reflex bradycardia, coronary artery occlusion, pulmonary embolism. , Overdose Symptoms: , restlessness, , nervousness, , dizziness, , sweating, , vomiting, , palpitations, weak or shallow breathing. Treatment: if systemic action of phenylephrine occurs, undesirable effects can be stopped by administering alpha-blockers, for example, 5-10 mg of phentolamine intravenously. If necessary, you can repeat the injection. , , Interaction with other drugs The mydriatic effect of phenylephrine is enhanced when used in combination with topical atropine. Due to the increased vasopressor action, tachycardia may develop. The use of phenylephrine with monoamine oxidase inhibitors, as well as within 21 days after stopping their use, should be carried out with caution, since in this case there is the possibility of an uncontrolled rise in blood pressure. The vasopressor effect of α-adrenomimetics can also be potentiated when used simultaneously with tricyclic antidepressants, propranolol, reserpine, guanethidine, methyldopa and m-anticholinergics. Phenylephrine may potentiate depression of cardiovascular activity during inhalation anesthesia. Concomitant use with other adrenergic agonists and sympathomimetics may enhance the effect of phenylephrine on the cardiovascular system. The use of phenylephrine may cause a weakening of concomitant antihypertensive therapy and lead to an increase in blood pressure and tachycardia. , Special instructions Exceeding the recommended dose of 2.5% solution in patients with injuries, diseases of the eye or its appendages, in the postoperative period, or with reduced tear production (anesthesia), may lead to an increase in , absorption, phenylephrine, and the development of systemic side effects. , Impact on the ability to drive vehicles and mechanisms After , the use of , the drug , due to , changes in accommodation and pupil width , there may be a decrease in visual acuity , therefore , until , its , recovery , it is not recommended to drive , transport , means, and , engage in , potentially , dangerous , activities that require , increased , concentration , attention , and , speed of psychomotor reactions . , , Release form Eye drops, 2.5%. 5 ml, 10 ml in dropper bottles made of high-pressure polyethylene with a screw neck, a dropper stopper made of low-pressure polyethylene and a screw-on cap with a control , ring , first , opening , , , from , low-density polyethylene. , 1, a dropper bottle, polymer, together with, instructions for, use, are placed in a pack of cardboard. , Storage conditions Store in a place protected from light at a temperature not exceeding 25 ̊ C. , Keep out of the reach of children. , Shelf life 2 years. Do not use the drug after the expiration date indicated on the package. Store an opened dropper bottle for no more than 30 days. , Dispensing conditions Dispensed by prescription. , Name , and , address , of the manufacturer/organization, , accepting , consumer claims, CJSC "LECCO", , Russia, 601125, Vladimir region, Petushinsky district, pos. Volginsky, tel./fax (49 243) 71 5 52. , , , , , ,