Betaxolol-Solopharm 0.5% eye drops dropper bottle 5ml
A country
Russia
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Compound
5 ml bottle
Betaxolol hydrochloride 5.6 mg per 1 ml. Excipients: benzalkonium chloride - 0.1 mg, sodium chloride - 5.49 mg, disodium phosphate dihydrate - 3.579 mg, disodium edetate (Trilon B) - 0.5 mg, sodium dihydrogen phosphate dihydrate - 3.165 mg, water for injection - up to 1 ml. Eye drops in the form of a clear, colorless or light yellow liquid.
pharmachologic effect
Cardioselective beta1-blocker without intrinsic sympathomimetic activity. Has weak membrane stabilizing activity. It has a hypotensive effect associated with a decrease in cardiac output and a decrease in sympathetic stimulation of peripheral vessels. When used in therapeutic doses, it does not have a cardiodepressive effect, does not affect glucose metabolism, does not reduce the bronchodilatory effect of beta-adrenergic agonists, and does not cause sodium ion retention in the body. Lasts for a long time. When applied topically in the form of eye drops, it reduces elevated intraocular pressure. The resorptive effect is slightly expressed.
Indications for use
For systemic use: as monotherapy and as part of combination therapy - arterial hypertension, prevention of angina attacks. For local use in ophthalmology: chronic open-angle glaucoma, increased intraocular pressure, condition after laser trabeculoplasty.
Side effects
From the cardiovascular system: at the beginning of treatment - AV block, sinus bradycardia, arterial hypotension, heart failure, Raynaud's syndrome. From the digestive system: rarely - abdominal pain, nausea, vomiting. From the central nervous system and peripheral nervous system: at the beginning of treatment - asthenia, paresthesia of the extremities, sleep disturbances, depression, drowsiness, dizziness. From the respiratory system: rarely - bronchospasm. Allergic reactions: rarely - psoriasis-like skin manifestations. Local reactions: - when used in the form of eye drops immediately after instillation, short-term discomfort in the eyes and sometimes lacrimation are possible; - rarely - decreased sensitivity of the cornea, erythema, itching, spotty coloration of the cornea, keratitis, anisocoria, photophobia.
Contraindications
Cardiogenic shock;
acute heart failure, chronic heart failure in the stage of decompensation, not compensated by treatment with diuretics, inotropes, ACE inhibitors, and other vasodilators; AV block II and III degrees (without an installed artificial pacemaker); Prinzmetal's angina (monotherapy is contraindicated); SSSU, sinoatrial block; severe bradycardia (heart rate less than 45-50 beats/min); severe peripheral circulatory disorders, severe forms of bronchial asthma and COPD; severe forms of Raynaud's disease and peripheral arterial occlusive disease; pheochromocytoma without simultaneous use of alpha-blockers; arterial hypotension (systolic blood pressure) Use during pregnancy and breastfeeding During pregnancy and lactation (breastfeeding), the use of betaxolol is possible only in cases where the expected benefit to the mother outweighs the possible risk to the fetus or child.
Use in children It is not recommended to use betaxolol in children.
Mode of application
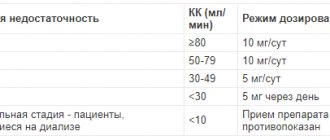
The method of administration and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. The compliance of the dosage form of a particular drug with the indications for use and dosage regimen should be strictly observed. For systemic use when taken orally - 20 mg 1 time / day. For patients on continuous hemodialysis or peritoneal dialysis, the initial dose is 10 mg/day; The time for taking betaxolol is set regardless of the mode of dialysis sessions. For local use in ophthalmology - 1 drop 2 times a day into the affected eye. During the first month, therapy is carried out under the control of the level of intraocular pressure; subsequently, the frequency of measuring intraocular pressure is determined individually. If betaxolol is used after previous treatment with another similar drug, the dosage regimen is determined individually.
special instructions
It should be used with caution in case of bronchial asthma and moderate COPD (start treatment with small doses and preferably under the control of respiratory function indicators; due to the beta1-selectivity of betaxolol, if an attack of bronchial asthma occurs while taking it, it is possible to relieve the attack with beta2-adrenergic agonists); in case of chronic heart failure in the compensation stage (treatment with betaxolol is possible only under strict medical supervision; treatment should begin with very small doses with a gradual increase); with AV blockade of the first degree (careful monitoring is required, including ECG monitoring); with obliterating diseases of peripheral arteries, Raynaud's syndrome (with the exception of severe forms) (possible increase in peripheral circulatory disorders); with Prinzmetal's angina (possible increased frequency of angina attacks; the use of a selective beta1-blocker is possible only with the simultaneous use of vasodilators); with treated pheochromocytoma (careful monitoring of blood pressure levels is required); in elderly patients (treatment should be started with small doses and under close medical supervision); in case of renal failure (with CC more than 20 ml/min - careful monitoring of the patient during the first few days of treatment; with CC less than 20 ml/min and/or hemodialysis, adjustment of the dosage regimen is required); with liver failure (more careful clinical monitoring is required at the beginning of treatment); in patients with diabetes mellitus (regular monitoring of blood glucose concentrations is required, including active self-monitoring by the patient at the beginning of treatment; it is possible to reduce the severity of precursors of hypoglycemia, such as tachycardia, palpitations and increased sweating); for psoriasis (beta-blockers can worsen the course of psoriasis); during desensitizing therapy. Betaxolol should be discontinued gradually, especially in patients suffering from coronary artery disease and angina pectoris. Betaxolol does not affect the size of the pupil, therefore, in case of angle-closure glaucoma, the drug should be used only in combination with miotics. When transferring a patient to betaxolol after treatment with several antiglaucoma drugs, the latter are discontinued gradually, over a period of at least 1 week per drug. With the simultaneous use of betaxolol in the form of eye drops and beta-blockers orally, additive effects may develop both on the part of intraocular pressure and manifestations of the systemic action of beta-blockers. Before a planned operation, beta-blockers, incl. betaxolol should be discontinued. When using betaxolol topically, you should not wear contact lenses. It is not recommended to use betaxolol in children. Effect on the ability to drive vehicles and operate machinery. Use with caution in patients whose activities require increased attention and rapid psychomotor reactions.
Interaction with other drugs
When used simultaneously with adrenergic agonists and xanthine derivatives, the effectiveness of betaxolol decreases. When used simultaneously with antacids and antidiarrheals, the absorption of beta-blockers may be reduced. When used simultaneously with antihypertensive drugs, the antihypertensive effect is enhanced. With the simultaneous use of halogen-containing agents for inhalation anesthesia, the negative inotropic effect may be enhanced. With simultaneous use of non-depolarizing muscle relaxants, their duration of action may be increased. With the simultaneous use of NSAIDs and corticosteroids, the antihypertensive effect of betaxolol decreases. With simultaneous use of cardiac glycosides, bradycardia may increase. With the simultaneous use of tricyclic antidepressants (imipramine), blood pressure decreases and there is a risk of developing orthostatic hypotension. With the simultaneous use of amiodarone, verapamil, diltiazem, beta-blockers for topical use in glaucoma, increased negative inotropic effects and conduction disturbances are possible. With simultaneous use of lidocaine, the concentration of lidocaine in the blood plasma increases. When used simultaneously with drugs that deplete catecholamine reserves (including reserpine), the hypotensive effect and bradycardia may be enhanced. When used simultaneously with sulfasalazine, the concentration of betaxolol in the blood plasma increases.
Dispensing conditions in pharmacies
On prescription
Betaxolol-Optic
If you missed taking Betaxolol-Optic, you should take it as soon as possible. Do not take the missed dose if it is near the time of your next dose. The next dose should be taken at the appropriate time in accordance with the treatment regimen. Do not double the dose.
Betaxolol does not affect the size of the pupil, therefore, in case of angle-closure glaucoma, the drug should be used only in combination with miotics as a means of lowering intraocular pressure.
Diabetes
Beta-blockers should be prescribed with caution to patients with a tendency to spontaneous hypoglycemia and patients with labile diabetes mellitus, since these drugs may mask the signs and symptoms of acute hypoglycemia.
Thyrotoxicosis
β-blockers may mask some symptoms of hyperthyroidism (eg, tachycardia). In patients with suspected thyrotoxicosis, β-blockers should not be abruptly discontinued, as this may cause an increase in symptoms.
Myasthenia gravis
β-blockers may cause symptoms and signs similar to those of myasthenia gravis (eg, diplopia, ptosis, general weakness).
Surgery
If surgery is necessary, the anesthesiologist should be informed that the patient is taking betaxolol. Before elective surgery, β-blockers should be gradually (not simultaneously!) withdrawn 48 hours before general anesthesia, since during general anesthesia they can reduce the sensitivity of the myocardium to symptomatic stimulation necessary for cardiac function (for example, they can block the action of systemic β -adrenaline agonist).
Pulmonology
Caution should be exercised when prescribing β-blockers to patients with reduced respiratory system function. Despite the fact that clinical studies have shown no effect of betaxolol on respiratory function, the possibility of increased sensitivity to the drug should not be excluded.
Risk of developing an anaphylactic reaction
Patients taking β-blockers may have a history of atopy or anaphylactic reactions. In case of repeated reactions, such patients may not be sensitive to the usual doses of epinephrine required to relieve anaphylaxis.
The drug should be used with caution in patients with severe peripheral circulatory disorders (ie, Raynaud's syndrome and pheochromocytoma).
When administered locally, β-blockers can enter the systemic circulation.
Thus, beta-blockers can cause cardiovascular, pulmonary and other adverse reactions, as with intravenous and parenteral administration. Cases of severe respiratory and cardiovascular disorders have been described, including death from bronchospasm in patients with bronchial asthma and death from heart failure.
Heart disorders
In patients with cardiovascular disease (eg, coronary artery disease, Prinzmetal's angina, heart failure) and hypotension, beta-blocker therapy should be critically evaluated and treatment with other active agents considered. Patients suffering from cardiovascular diseases should be closely monitored for signs of exacerbation of the disease and adverse reactions.
Corneal diseases
β-blockers may cause dry eyes. The drug should be used with caution in patients with corneal diseases.
Choroidal detachment
Cases of detachment of the choroid have been described when using drugs that prevent the formation of intraocular fluid (for example, timolol, acetazolamide) after filtering surgery.
Since the drug contains benzalkonium chloride, patients are not recommended to use soft (hydrophilic) contact lenses while using the drug. Contact lenses should be removed before instillation of the drug and put back on no earlier than 15 minutes later. Benzalkonium chloride may cause eye irritation and discolor contact lenses.