Composition per tablet:
| Active ingredient, mg: | ||
| Levofloxacin hemihydrate (in terms of levofloxacin) | 256,23 250,00 | 512,46 500,00 |
| Excipients, mg: | ||
| Lactitol | 300,00 | 600,00 |
| Crospovidone | 32,50 | 65,00 |
| Povidone K-17 | 10,00 | 20,00 |
| Sodium stearyl fumarate | 9,75 | 19,50 |
| Talc | 6,50 | 13,00 |
| Microcrystalline cellulose to obtain a tablet weight | 650,00 | 1300,00 |
| Excipients of the shell, mg: | until you get a tablet weighing | |
| 670,00 | 1340,00 | |
| Hypromellose | 9,52 | 19,04 |
| Titanium dioxide | 5,22 | 10,44 |
| Macrogol-4000 | 3,744 | 7,488 |
| Talc | 1,10 | 2,20 |
| Povidone K-17 | 0,416 | 0,832 |
Description
Capsule-shaped biconvex tablets, film-coated, white or almost white; on the cross section two layers are visible, the inner layer is light yellow to yellow in color, white inclusions are allowed.
Pharmacotherapeutic group:
antimicrobial agent, fluoroquinolone.
ATX code:
J01MA12.
Pharmacological properties
Pharmacodynamics
Levofloxacin is a synthetic broad-spectrum antibacterial drug from the group of fluoroquinolones, containing the levorotatory isomer of ofloxacin as an active substance.
Levofloxacin blocks DNA gyrase, disrupts supercoiling and cross-linking of DNA breaks, inhibits DNA synthesis, and causes profound morphological changes in the cytoplasm, cell wall and membranes of bacteria.
Levofloxacin has a bactericidal effect and is active against a large number of pathogens of bacterial infections both in vitro
, and
in vivo
.
Sensitive microorganisms (minimum inhibitory concentration (MIC) ≤ 2 mg/l):
- aerobic gram-positive microorganisms: Bacillus anthracis , Corynebacterium diphtheriae , Corynebacterium jeikeium , Enterococcus spp
., including
Enterococcus faecalis, Listeria monocytogenes, Staphylococcus spp.
( coagulase-negative, methicillin-sensitive/leukotoxin-containing/moderately sensitive strains), including
Staphylococcus aureus (
methicillin-sensitive strains
), Staphylococcus epidermidis (
methicillin-sensitive strains
), Streptococcus spp.
groups C and G, Streptococcus agalactiae, Streptococcus pneumoniae ( penicillin-sensitive / moderately sensitive / resistant strains
), Streptococcus pyogenes, Streptococcus spp.
Viridans group ( penicillin-sensitive/resistant strains
); - aerobic gram-negative microorganisms: Acinetobacter spp .
, including
Acinetobacter baumannii , Acinetobacillus actinomycetemcomitans , Citrobacter freundii , Eikenella corrodens , Enterobacter spp
., including
Enterobacter aerogenes , Enterobacter cloacae , Escherichia coli , Gardnerella vaginalis , Haemophilus ducreyi , Haemophilus influenzae
(ampicillin-sensitive/ resistant strains),
Haemophilus parainfluenzae , Helicobacter pylori , Klebsiella spp
., including
Klebsiella oxytoca , Klebsiella pneumoniae , Moraxella catarrhalis
(beta-lactamase producing and non-producing strains),
Morganella morganii , Neisseria gonorrhoeae
(penicillinase producing and non-producing strains),
Neisseria meningitidis , Pasteurella spp .
, including
Pasteurella canis , Pasteurella dagmatis , Pasteurella multocida , Proteus mirabilis , Proteus vulgaris , Providencia spp
., including
Providencia rettgeri , Providencia stuartii , Pseudomonas spp .
, including
Pseudomonas aeruginosa
(hospital infections caused by
Pseudomonas aeruginosa
may require combination treatment),
Serratia spp
., including
Serratia marcescens , Salmonella spp
.; - anaerobic microorganisms: Bacteroides fragilis , Bifidobacterium spp ., Clostridium perfringens , Fusobacterium spp ., Peptostreptococcus spp ., Propionibacterium spp ., Veillonella spp
.; - other microorganisms: Bartonella spp ., Chlamydia pneumoniae , Chlamydia psittaci , Chlamydia trachomatis , Legionella pneumophila , Legionella spp ., Mycobacterium spp .
, including
Mycobacterium leprae , Mycobacterium tuberculosis , Mycoplasma hominis , Mycoplasma pneumoniae , Rickettsia spp ., Ureaplasma urealyticum
.
Moderately sensitive microorganisms (MIC = 4 mg/l):
- aerobic gram-positive microorganisms: Corynebacterium urealyticum , Corynebacterium xerosis , Enterococcus faecium , Staphylococcus epidermidis
(methicillin-resistant strains),
Staphylococcus haemolyticus
(methicillin-resistant strains); - aerobic gram-negative microorganisms: Campylobacter jejuni , Campylobacter coli
; - anaerobic microorganisms: Prevotella spp ., Porphyromonas spp
.
Resistant microorganisms (MIC more than 8 mg/l):
- aerobic gram-positive microorganisms: Staphylococcus aureus
(methicillin-resistant strains), other
Staphylococcus spp
. (coagulase-negative methicillin-resistant strains); - aerobic gram-negative microorganisms: Alcaligenes xylosoxidans
; - anaerobic microorganisms: Bacteroides thetaiotaomicron
- other microorganisms: Mycobacterium avium
.
Clinical efficacy (effectiveness in clinical studies against infections caused by the following microorganisms):
- aerobic gram-positive microorganisms: Enterococcus faecalis , Staphylococcus aureus , Streptococcus pneumoniae , Streptococcus pyogenes
; - aerobic gram-negative microorganisms: Citrobacter freundii , Enterobacter cloacae , Escherichia coli , Haemophilus influenzae , Haemophilus parainfluenzae , Klebsiella pneumoniae , Moraxella catarrhalis , Morganella morganii , Proteus mirabilis , Pseudomonas aeruginosa , Serratia marcescens
; - other microorganisms: Chlamydia pneumoniae , Legionella pneumophila , Mycoplasma pneumoniae
.
Resistance to levofloxacin develops as a result of a stepwise process of mutations in the genes encoding both type II topoisomerases: DNA gyrase and topoisomerase IV. Other resistance mechanisms, such as the mechanism of influencing the penetration barriers of the microbial cell (a mechanism characteristic of Pseudomonas
aeruginosa
) and the mechanism of efflux (active removal of the antimicrobial agent from the microbial cell), may also reduce the sensitivity of microorganisms to levofloxacin.
Due to the peculiarities of the mechanism of action of levofloxacin, cross-resistance between levofloxacin and other antimicrobial agents is not usually observed.
Pharmacokinetics
Absorption
Levofloxacin is rapidly and almost completely absorbed after oral administration; food intake has little effect on its absorption. Absolute bioavailability when taken orally is 99-100%. After a single dose of 500 mg of levofloxacin, the maximum concentration in blood plasma (Cmax) is reached within 1-2 hours and is 5.2 ± 1.2 μg/ml. The pharmacokinetics of levofloxacin is linear in the dose range from 50 to 1000 mg. The equilibrium state of levofloxacin concentration in blood plasma when taking 500 mg of levofloxacin 1 or 2 times a day is achieved within 48 hours.
On the 10th day of oral administration of the drug Levofloxacin Ecolevid® 500 mg 1 time per day, the Cmax of levofloxacin was 5.7 ± 1.4 mcg/ml, and the minimum concentration of levofloxacin (concentration before taking the next dose) (Cmin) in the blood plasma was 0.5 ±0.2 µg/ml.
On the 10th day of oral administration of the drug Levofloxacin Ecolevid® 500 mg 2 times a day, Cmax was 7.8 ± 1.1 μg/ml, and C min was 3.0 ± 0.9 μg/ml.
Distribution
The connection with serum proteins is 30-40%. After a single and repeated dose of 500 mg of levofloxacin, the volume of distribution of levofloxacin is, on average, 100 l, which indicates good penetration of levofloxacin into organs and tissues of the human body.
Penetration into the bronchial mucosa, epithelial lining fluid, alveolar macrophages
After a single oral dose of 500 mg of levofloxacin, the maximum concentrations of levofloxacin in the bronchial mucosa and epithelial lining fluid were reached within 1 hour or 4 hours and were 8.3 μg/g and 10.8 μg/ml, respectively, with penetration coefficients into the mucosa bronchi and epithelial lining fluid, compared with plasma concentrations of 1.1-1.8 and 0.8-3, respectively.
After 5 days of oral administration of 500 mg levofloxacin, the mean concentrations of levofloxacin 4 hours after the last dose in the epithelial lining fluid were 9.94 μg/ml and in alveolar macrophages - 97.9 μg/ml.
Penetration into lung tissue
Maximum concentrations in lung tissue after oral administration of 500 mg of levofloxacin were approximately 11.3 mcg/g and were achieved 4-6 hours after dosing with penetration coefficients of 2-5, compared with plasma concentrations.
Penetration into alveolar fluid
After 3 days of taking 500 mg of levofloxacin 1 or 2 times a day, the maximum concentrations of levofloxacin in the alveolar fluid were reached 2-4 hours after taking the drug and were 4.0 and 6.7 μg/ml, respectively, with a penetration coefficient of 1. compared to plasma concentrations.
Penetration into bone tissue
Levofloxacin penetrates well into cortical and cancellous bone tissue in both the proximal and distal parts of the femur, with a penetration coefficient (bone tissue/blood plasma) of 0.1-3. The maximum concentrations of levofloxacin in the cancellous bone tissue of the proximal femur after oral administration of 500 mg of the drug were approximately 15.1 mcg/g (2 hours after dosing).
Penetration into the cerebrospinal fluid
Levofloxacin penetrates poorly into the cerebrospinal fluid.
Penetration into prostate tissue
After oral administration of 500 mg of levofloxacin once daily for 3 days, the average concentration of levofloxacin in prostate tissue was 8.7 mcg/g, the average prostate/blood plasma concentration ratio was 1.84.
Concentrations in urine
Mean urinary concentrations 8 to 12 hours after oral doses of 150, 300, and 600 mg of levofloxacin were 44 mcg/mL, 91 mcg/mL, and 162 mcg/mL, respectively.
Metabolism
Levofloxacin is metabolized to a small extent (5% of the dose taken). Its metabolites are demethyllevofloxacin and levofloxacin N-oxide, which are excreted by the kidneys. Levofloxacin is stereochemically stable and does not undergo chiral transformations.
Removal
After oral administration, levofloxacin is relatively slowly eliminated from the blood plasma (half-life (T1/2) - 6-8 hours). Excretion is mainly through the kidneys (more than 85% of the dose taken). The total clearance of levofloxacin after a single dose of 500 mg was 175±29.2 ml/min.
There are no significant differences in the pharmacokinetics of levofloxacin when administered intravenously and orally, which confirms that oral and intravenous administration are interchangeable.
Pharmacokinetics in selected patient groups
The pharmacokinetics of levofloxacin do not differ between men and women.
Pharmacokinetics in elderly patients do not differ from those in younger patients, with the exception of differences in pharmacokinetics associated with differences in creatinine clearance (CC).
In renal failure, the pharmacokinetics of levofloxacin changes. As renal function deteriorates, renal excretion and renal clearance (CIR) decrease and T1/2 increases.
Pharmacokinetics in renal failure after a single oral dose of 500 mg of Levofloxacin Ecolevid®.
| CC (ml/min) | <20 | 20-49 | 50-80 |
| CIR (ml/min) | 13 | 26 | 57 |
| T1/2 (h) | 35 | 27 | 9 |
Fluoroquinolones, first approved for medical use in the 1980s, are among the most widely used antibacterial drugs. The combination of basic pharmacological properties (a wide spectrum of antimicrobial activity, an original mechanism of action, favorable pharmacokinetic properties and good tolerability) serves as the basis for their use in a wide range of community-acquired and nosocomial infections.
The introduction of various halogens and substituent groups into different positions of the quinolone core made it possible to synthesize a variety of drugs from the fluoroquinolone group of four generations with different benefit-risk ratios. The development of some representatives of this class was stopped at the pre-marketing stage, others were withdrawn in the post-marketing period due to safety problems, for example, tovafloxacin (hepatotoxicity) and grepafloxacin (development of life-threatening cardiac arrhythmias). A number of fluoroquinolones, in particular lomefloxacin and sparfloxacin, which have a higher risk of photo- and cardiotoxicity, have been displaced from the market in many countries by other drugs with a better benefit-risk ratio. Thus, only five fluoroquinolones remain on the US pharmaceutical market, and the volume of their prescriptions in recent years shows that some of them are also losing their clinical significance (Fig. 1). The only fluoroquinolone whose prescriptions have remained stable over the years is ciprofloxacin, and the only drug whose prescriptions continue to increase is levofloxacin [1]. Of the approximately 33 million fluoroquinolone prescriptions written in the United States in 2014, these 2 drugs accounted for approximately 22 million (ciprofloxacin - approximately 20 million, levofloxacin - 11.3 million), while the number of prescriptions for gemifloxacin was only 7006 , ofloxacin – 9500, and moxifloxacin – 609 thousand. A similar situation is observed in European countries, where prescriptions of ciprofloxacin and levofloxacin exceed 50% (median – 73%) of the prescriptions of all fluoroquinolones [2].
The basis for the increase in consumption of levofloxacin is its more favorable benefit-risk ratio compared to many other fluoroquinolones, as evidenced by the results of not only clinical, but also widely ongoing pharmacoepidemiological studies.
Pharmacological properties of levofloxacin
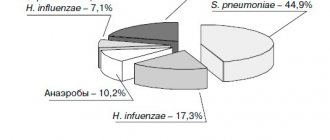
Levofloxacin has a wide spectrum of antibacterial action, including a large number of gram-positive and gram-negative aerobic microorganisms, incl. intracellular bacteria (Mycoplasma pneumonia, Chlamydia pneumoniae and Legionella pneumophila), Mycobacterium tuberculosis and Pseudomonas aeruginosa. It belongs to the so-called. respiratory fluoroquinolones, which are highly effective against the main pathogens of upper and lower respiratory tract infections, incl. Streptococcus pneumoniae, resistant to penicillins and macrolides. Levofloxacin is active against pathogens of the Enterobacteriacae family and, unlike most other drugs in its group, exhibits antipseudomonas activity, which serves as the basis for its prescription for nosocomial infections, incl. caused by multi-resistant microorganisms.
Levofloxacin is characterized by excellent pharmacokinetic properties, absolute (100%) bioavailability when taken orally, and a fairly long half-life (6–8 hours), providing high concentrations of the drug in the blood exceeding the MIC90 for many microorganisms, including the main causative agents of respiratory tract infections and pathogens of the Enterobacteriacae family. , for more than 24 hours, a large volume of distribution (90–110 l) and good penetration into the ENT organs, lungs, bronchial mucosa, sputum, bronchoalveolar fluid, prostate gland and prostatic fluid, gynecological organs, liver, biliary tract, skin , bones and joints. Excreted primarily by the kidneys, it creates high concentrations in the urine. The drug penetrates well into phagocytes and accumulates intracellularly [2].
The combination of a wide spectrum of action and pharmacokinetic properties ensures the effectiveness of levofloxacin for infections of various localizations, including nosocomial and community-acquired pneumonia, infections of the upper respiratory tract, skin and soft tissues, and urinary tract; chronic prostatitis and drug-resistant tuberculosis.
Safety and tolerability of levofloxacin
In recent decades, increasing attention has been paid to the safety issues of fluoroquinolones, which have a significant impact on the choice of a particular drug for a particular patient. Thanks to the development of pharmacovigilance systems in different countries and the introduction of electronic patient databases, the ability to assess the safety and tolerability of drugs not only in clinical trials, but also in real clinical practice has significantly expanded. Below are data on the safety of levofloxacin in comparison with other antibacterial agents, primarily other fluoroquinolone drugs, as well as risk factors for the development of rare serious adverse reactions (ARs) of drugs in this group, which must be taken into account to improve patient safety.
In clinical studies involving approximately 7000 patients with skin, respiratory and urinary tract infections, levofloxacin was at least as tolerable as amoxicillin/clavulanate, imipenem, clarithromycin, azithromycin, ceftriaxone, cefuroxime axetil, cefdinir and cefaclor [3], and the frequency of its serious ADRs in real medical practice per 1 million prescriptions in the first 39 months of being on the US market (15 million prescriptions) was: tendon ruptures - 4, taste disturbances - 3, convulsions - 2, photosensitivity - 1, hepatitis - 1 , liver failure – 1, QT interval prolongation – 1, torsade de pointes – 1, empyema – 1.7 [4]. An analysis of the Italian Pharmacovigilance database also showed that the relative risk of developing adverse reactions with levofloxacin, ciprofloxacin and norfloxacin did not differ from that for other classes of oral antibiotics, while cinoxacin and moxifloxacin were associated with a higher risk [5].
In clinical trials of levofloxacin, the most common adverse event was diarrhea, occurring in 4–6%, which is significantly lower than with oral penicillins or cephalosporins [6]. Adverse reactions from the central nervous system (headache, dizziness and sleep disturbances), which are a class effect of fluoroquinolones, were observed less frequently during treatment with levofloxacin (1%) than during treatment with gatifloxacin and moxifloxacin (2–3%).
Serious adverse reactions from the central nervous system, in particular seizures when using any fluoroquinolones, including levofloxacin, mainly occur in patients with risk factors for their development: epilepsy, traumatic brain injury, hypoxia, metabolic disorders, and self-resolve after discontinuation of the drug [7]. A risk factor for the development of seizures is also the concomitant use of non-steroidal anti-inflammatory drugs.
Another potential risk common to all fluoroquinolones is phototoxicity. The highest potential for phototoxicity is possessed by drugs that have a halogen atom in the 8th position of the quinolone ring - fleroxacin, clinafloxacin, sparfloxacin and lomefloxacin [8]. Thus, when using the last two drugs it is 8–10% or more [7, 9], while when using levofloxacin it does not exceed 0.1% [8].
The incidence of skin rashes with levofloxacin is 0.2% compared to 2.0% with moxifloxacin, 2.8–4.8% with gemifloxacin, 5.1% with sparfloxacin [8, 10]. Serious allergic reactions with its use are extremely rare. Over the almost 20-year period of widespread use of levofloxacin in real medical practice, only 4 cases of toxic epidermal necrolysis have been published [10]. The frequency of anaphylactoid reactions during treatment with levofloxacin is comparable to that with the use of most drugs in this group - 1.8–23 per 1 million days of treatment [10], and, apparently, significantly lower than when using moxifloxacin [11, 12]. Thus, according to the German Pharmacovigilance authorities, among 166 spontaneous reports of anaphylaxis with fluoroquinolones, 54% were associated with the use of moxifloxacin [13]. The incidence of anaphylaxis per 1 million daily doses was 3.3 for moxifloxacin, while for levofloxacin it was 0.6, and for ciprofloxacin and ofloxacin it was 0.2 each. It should be noted that some experts associate the increase in the frequency of hypersensitivity reactions to fluoroquinolones in the last decade with the introduction of moxifloxacin into medical practice [11, 12].
Hepatotoxic reactions with the use of fluoroquinolones are predominantly idiosyncratic (independent of dose and unpredictable) in nature. An increase in AST and ALT levels is observed in 2–3% of patients receiving drugs in this group.
In clinical studies of levofloxacin, hepatobiliary lesions (impaired liver function, increased levels of liver enzymes and alkaline phosphatase) occurred in 0.1–1% of cases [10]. The incidence of hepatitis, liver necrosis and liver failure during treatment with levofloxacin, according to French Pharmacovigilance data, is <1 5=»» 14=»» fda=»» 2=»» 1=»» 10=»» 58=»» 10= »» 6=»» 6=»» 6=»» 0=»» 15=»» 9=»» 16=»» 2008=»» ema=»» 2010=»» 17=»» 18=»» p=»»>
Fluoroquinolones are among the antibacterial agents associated with the highest risk of musculoskeletal injury, possibly due to their ability to chelate with di- and trivalent cations and have a toxic effect on collagen. When used, damage to tendons, cartilage, bones and muscles is possible.
Diffuse muscle pain, often in combination with muscle weakness, usually occurs in the first week of treatment and in most cases resolves spontaneously within 1–4 weeks after discontinuation of fluoroquinolones. However, there are reports of persistence of muscle pain for 6 months or more [19]. The risk of severe muscle damage, including rhabdomyolysis, increases with concomitant use of statins [20, 21], as well as in patients with underlying myopathy and myasthenia gravis [22, 23]. When using fluoroquinolones in patients with myasthenia, severe exacerbations have been described, incl. with fatal outcomes [23]. Comparative data on the incidence of muscle lesions with the use of different fluoroquinolone preparations could not be found in the available literature.
Concerns about the chondrotoxic effects of fluoroquinolones are based on experimental studies that have shown irreversible cartilage damage in fast-growing postnatal animals. Data on the development of arthropathy in humans are contradictory, incl. due to the lack of a unified definition of the term “arthropathy” and its use to describe various adverse reactions from the musculoskeletal system [6]. The results of numerous magnetic resonance imaging studies are inconsistent and difficult to interpret due to possible confounding factors, and there are no targeted randomized clinical trials examining chondrotoxicity in children.
Data from epidemiological studies are also contradictory. An increased risk of joint damage with fluoroquinolones was not observed in three of the four retrospective studies reviewed by Forsythe and Ernst (2007), although one of these studies found a correlation between pefloxacin use and the development of arthropathies [24]. A five-year follow-up of children (n=6000) taking antibiotics showed that the incidence of tendon or joint injuries with levofloxacin, ofloxacin or ciprofloxacin was <1% and comparable to that with azithromycin [25]. A systematic review assessing the efficacy and safety of ciprofloxacin in neonates found no evidence of osteoarticular toxicity [26]. Levofloxacin, according to the results of an analysis of clinical studies involving children older than 6 months with community-acquired pneumonia and otitis media, more often (1.6%) caused musculoskeletal lesions than β-lactam antibiotics and macrolides (0.7%), but the resulting The results may have been influenced by the inclusion of children with underlying joint diseases in these studies [27].
Thus, the available data do not allow us to unequivocally confirm the presence of an increased risk of arthropathy in children when treated with the most widely used fluoroquinolone drugs. However, childhood and pregnancy are contraindications to the use of fluoroquinolones.
In adult patients, fluoroquinolone-associated arthralgia is estimated to occur in 1% of cases, but its exact prevalence is unknown. The relationship between arthralgia and the presence of initial structural changes in the patient also remains unclear. There have been no studies assessing the risk of developing arthropathy itself in adult patients.
Tendinopathy (tendonitis and rupture of the tendon, mainly Achilles) has been described during treatment with all fluoroquinolone preparations for systemic use. According to systematic reviews, tendinopathy is slightly more common with ofloxacin, pefloxacin and ciprofloxacin than with other drugs in this group [28, 29]. The risk of their development when using ofloxacin and levofloxacin depends on the dose and duration of use [30].
In epidemiological studies, the incidence of tendinopathies with the use of fluoroquinolones was 1:2000 [6], according to the FDA - 1.3-5.6 per 10 thousand patient-years [31]. However, in patients with risk factors (see table), the likelihood of their development increases significantly. For example, in people who have undergone a kidney transplant, it can reach 12.2–15.6% [32], and in people over 80 years of age it can increase by 20.4 times [33]. If adequate therapeutic measures are not taken at the first symptoms, the lesion often progresses to tendon rupture. Approximately half of patients with tendon rupture had a recent history of glucocorticosteroid use [28].
Experiments on animals revealed the inhibitory effect of fluoroquinolones on the healing of bone fractures [34]. The clinical significance of this finding remains unclear, but until it is clarified, the authors recommended against the use of fluoroquinolones in the perioperative period in patients undergoing arthroplasty.
Fluoroquinolones, along with macrolides and azoles, are among the antimicrobial drugs whose use is most often associated with prolongation of the QT interval on the ECG [36]. Significant prolongation of the QT interval contributes to the development of torsades de pointes, a potentially fatal polymorphic ventricular tachycardia.
Due to the increased risk of developing torsade de pointes, grepafloxacin and the original drug sparfloxacin were withdrawn from the global pharmaceutical market. The mechanism of QT interval prolongation is associated with blockade of hERG potassium channels ((human ether-a-go-go-related), primarily leading to inhibition of the fast component of the potassium current rectifier (IKr) from myocytes, accumulation of potassium ions in myocytes and slowing of ventricular repolarization .
Fluoroquinolone preparations differ in their ability to inhibit hERG channels and, as a consequence, in their ability to induce torsade de pointes [37]. According to the degree of inhibitory effect on hERG channels, fluoroquinolones can be ranked in the following order: sparfloxacin > grepafloxacin > moxifloxacin > gatifloxacin > levofloxacin > ciprofloxacin > ofloxacin, which coincides with the ranking of fluoroquinolones according to the frequency of recording QT prolongation on the ECG [10].
The risk of developing life-threatening arrhythmias with different fluoroquinolone drugs, according to the results of a population-based study conducted in Canada in 1990–2007. (1838 cases) [38], presented in Fig. 2.
The average QT prolongation caused by fluoroquinolones (3–6 ms) in patients with a normal QT interval (450–470 ms) is not of significant clinical significance. However, in individuals with a baseline QT interval >500 ms, fluoroquinolones should be avoided.
Risk factors for QT prolongation include:
- female;
- elderly age;
- hypokalemia, severe hypomagnesemia;
- bradycardia;
- recent cardioversion for atrial fibrillation, especially with QT prolonging drugs;
- chronic heart failure;
- left ventricular hypertrophy;
- ventricular arrhythmia;
- digoxin treatment;
- simultaneous use of 2 drugs that prolong QT;
- hereditary predisposition to QT prolongation [6, 40].
Since the degree of prolongation of the QT interval depends on the concentration of fluoroquinolones, the risk of arrhythmia increases under the influence of factors that increase the concentrations of these drugs in the blood or reduce their clearance, including. in case of impaired renal or liver function, against the background of drug interactions, with very rapid intravenous administration [6, 39].
Results from a recently published pilot study suggest that the risk of levofloxacin affecting the QT interval is dependent on circadian rhythms and may be reduced by adapting the timing of its administration to daily variations in cardiovascular parameters [40]. The maximum increase in the risk of QT interval prolongation was observed when the drug was administered at 2:00 p.m., the minimum at 6:00 a.m., however, these data require confirmation in further studies.
A number of studies have identified an association between the use of fluoroquinolones and disturbances in glucose homeostasis. The maximum risk of hypoglycemia is characteristic of gatifloxacin [41, 42]. In a comparative study, the risk of hypoglycemia with gatifloxacin was 2.81 times higher than that with levofloxacin [41]. In addition, gatifloxacin was associated with a high risk of hyperglycemia (odds ratio, 16.7), while no risk of this complication was identified with the use of other fluoroquinolones [42, 43]. The increased risk of dysglycemia, combined with the risk of hallucinations, drug-induced liver injury, and purpura, led to discontinuation of gatifloxacin in several countries [32].
The good tolerability of levofloxacin is also evidenced by the results of a pharmacoepidemiological study in the USA, which assessed the frequency of outpatient visits to medical care for ADRs of fluoroquinolones [44]. The estimated number of visits per year per 10 thousand population was minimal when using ciprofloxacin - 6.4 (95% confidence interval [CI] - 4.5-8.4), maximum when using moxifloxacin - 20.7 (95% CI – 11.9–29.5), during treatment with levofloxacin it was 8.9 (95% CI – 6.2–11.5).
Thus, levofloxacin has a wide spectrum of antibacterial activity against a variety of gram-positive and gram-negative microorganisms, including P. aeruginosa, excellent pharmacokinetic properties that allow it to achieve high concentrations in foci of infection of various locations and exhibit activity against intracellular pathogens, and good tolerability, at least not inferior to those of antibiotics from other widely used groups, and in many cases superior to it, which determines the favorable benefit-risk ratio of the drug for many community-acquired and nosocomial infections.
The risk of developing serious adverse reactions with levofloxacin is generally lower than with other fluoroquinolones and can be significantly reduced by rational use of the drug, which involves taking into account the risk factors for developing certain adverse reactions in a particular patient. The rational use of levofloxacin, as well as other fluoroquinolone drugs, will also reduce the rate of increase in antibiotic resistance to them, which is currently causing concern among the medical community. This is partly the focus of the FDA's recent warning to limit the use of fluoroquinolones for acute sinusitis, exacerbations of chronic bronchitis, and urinary tract infections for which alternative treatment options exist. In addition, the use of short 5-day courses of high dose levofloxacin (750 mg) is being considered as a measure to potentially reduce the risk of developing resistance [2, 45]. This treatment regimen allows you to create higher concentrations of the antibiotic in the blood, quickly eliminate the symptoms of infection and increase patient adherence to therapy. At the same time, the safety profile and frequency of side effects of levofloxacin when used in doses of 500 and 750 mg did not differ significantly in both clinical and pharmacoepidemiological studies.
In order to reduce the cost of treatment with levofloxacin, it is advisable to use its effective generics, in particular the drug Levolet R (Doctor Reddys Laboratories).
Indications for use
Treatment of infectious and inflammatory diseases caused by microorganisms sensitive to levofloxacin:
- community-acquired pneumonia;
- complicated urinary tract infections and pyelonephritis;
- chronic bacterial prostatitis;
- infections of the skin and soft tissues;
- for complex treatment of drug-resistant forms of tuberculosis;
- prevention and treatment of anthrax through airborne transmission.
For the treatment of the following infectious and inflammatory diseases, levofloxacin can be used as an alternative to other antimicrobial drugs:
- acute sinusitis;
- exacerbation of chronic bronchitis;
- uncomplicated cystitis.
When using the drug Levofloxacin Ecolevid®, official national recommendations on the proper use of antibacterial drugs, as well as the sensitivity of pathogenic microorganisms in a particular country should be taken into account (see section "Special instructions").
Levofloxacin
The frequency of a particular side effect is determined using the following table:
| Frequency | Occurrence of side effects |
| often: | in 1-10 patients out of 100 |
| Sometimes: | less than 1 patient out of 100 |
| rarely: | less than 1 patient out of 1,000 |
| very rarely: | less than 1 patient out of 10,000 |
| individual cases | less than 0.01% |
Allergic reactions: sometimes – itching and redness of the skin; rarely - general hypersensitivity reactions (anaphylactic and anaphylactoid reactions) with symptoms such as urticaria, constriction of the bronchi and possibly severe suffocation; very rarely - swelling of the skin and mucous membranes (for example, in the face and throat), sudden drop in blood pressure and shock, increased sensitivity to solar and ultraviolet radiation (see "Special Instructions"), allergic pneumonitis, vasculitis; in some cases - severe skin rashes with blistering, for example, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell's syndrome) and exudative erythema multiforme. General hypersensitivity reactions may sometimes be preceded by milder skin reactions. The above reactions can develop after the first dose, a few minutes or hours after administration of the drug.
From the digestive system: often - nausea, diarrhea, increased activity of liver enzymes (for example, alanine aminotransferase and aspartate aminotransferase); sometimes - loss of appetite, vomiting, abdominal pain, digestive disorders; rarely - diarrhea mixed with blood, which in very rare cases may be a sign of intestinal inflammation and even pseudomembranous colitis (see "Special Instructions").
On the metabolic side: very rarely - a decrease in the concentration of glucose in the blood, which is of particular importance for patients with diabetes (possible signs of hypoglycemia: increased appetite, nervousness, perspiration, trembling). Experience with the use of other quinolones suggests that they can cause exacerbation of porphyria in patients already suffering from this disease. A similar effect cannot be excluded when using the drug levofloxacin.
From the nervous system: sometimes - headache, dizziness and/or stupor, drowsiness, sleep disturbances; rarely - anxiety, paresthesia in the hands, trembling, psychotic reactions such as hallucinations and depression, agitation, convulsions and confusion; very rarely - impaired vision and hearing, impaired taste and smell, decreased tactile sensitivity.
From the cardiovascular system: rarely - increased heartbeat, decreased blood pressure; very rarely - vascular (shock-like) collapse; in some cases - prolongation of the QT interval.
From the musculoskeletal system: rarely - tendon damage (including tendonitis), joint and muscle pain; very rarely - tendon rupture (for example, Achilles tendon); this side effect can be observed within 48 hours after the start of treatment and can be bilateral (see “Special Instructions”), muscle weakness, which is of particular importance for patients with bulbar syndrome; in some cases - muscle damage (rhabdomyolysis).
From the urinary system: rarely - increased levels of bilirubin and creatinine in the blood serum; very rarely - deterioration of kidney function up to acute renal failure, interstitial nephritis.
From the hematopoietic organs: sometimes - an increase in the number of eosinophils, a decrease in the number of leukocytes; rarely - neutropenia, thrombocytopenia, which may be accompanied by increased bleeding; very rarely - agranulocytosis and the development of severe infections (persistent or recurrent increase in body temperature, deterioration in health); in some cases - hemolytic anemia; pancytopenia.
Other: sometimes - general weakness; very rarely - fever.
Any antibiotic therapy can cause changes in the microflora that is normally present in humans. For this reason, increased proliferation of bacteria and fungi resistant to the antibiotic used may occur, which in rare cases may require additional treatment.
Contraindications
Hypersensitivity to levofloxacin, other fluoroquinolones or components of the drug, epilepsy, tendon damage during previous treatment with quinolones, pregnancy, lactation, childhood and adolescence (up to 18 years), myasthenia gravis.
Lactose intolerance or lactase deficiency, as well as glucose-galactose malabsorption.
Due to the inability to split the tablet in two, the use of the drug is contraindicated in patients with impaired renal function:
- in patients with creatinine clearance less than 50 ml/min, it is impossible to use a dosage regimen with an initial dosage of 250 mg/24 hours;
- in patients with creatinine clearance less than 20 ml/min, it is impossible to use the dosage regimen with an initial dosage of 500 mg/24 hours and 500 mg/12 hours;
- when creatinine clearance is less than 10 ml/min (including during hemodialysis and continuous ambulatory peritoneal dialysis), it is impossible to use it for all dosage regimens.
Carefully
- In patients predisposed to the development of seizures [in patients with previous lesions of the central nervous system (CNS); in patients simultaneously taking drugs that lower the threshold of convulsive activity of the brain, such as fenbufen, theophylline] (see section “Interaction with other drugs”);
- In patients with latent or manifest deficiency of glucose-6-phosphate dehydrogenase (increased risk of hemolytic reactions during treatment with quinolones);
- In patients with impaired renal function (mandatory monitoring of renal function is required, as well as correction of the dosage regimen, see section “Dosage and Administration”);
- In patients with known risk factors for QT interval prolongation: in elderly patients; in female patients; in patients with uncorrected electrolyte disturbances (with hypokalemia, hypomagnesemia); with congenital long QT syndrome; with heart disease (heart failure, myocardial infarction, bradycardia); while taking medications that can prolong the QT interval (class IA and III antiarrhythmic drugs, tricyclic antidepressants, macrolides, antipsychotics) (see sections “Overdose”, “Interaction with other drugs”, “Special instructions”);
- In patients with diabetes mellitus receiving oral hypoglycemic drugs (for example, glibenclamide) or insulin drugs (the risk of hypoglycemia increases);
- In patients with severe adverse reactions to other fluoroquinolones, such as severe neurological reactions (increased risk of developing similar adverse reactions when using levofloxacin);
- In patients with psychosis or in patients with a history of mental illness (see section "Special instructions");
- In elderly patients, in patients after transplantation, as well as with concomitant use of glucocorticosteroids (increased risk of tendinitis and tendon rupture) (see section "Special instructions").
Fluoroquinolone antibiotics: dangerous and unpredictable
The US Food and Drug Administration (FDA) has warned that fluoroquinolone antibacterial drugs carry an increased risk of the rare but serious adverse reactions of ruptured aortic aneurysm or aortic dissection. Such phenomena can lead to heavy bleeding and death. The information letter indicates these risks in the case of the use of both oral and injectable systemic fluoroquinolones.
According to the regulator's recommendations, fluoroquinolones should not be prescribed to patients with an increased risk of these adverse events unless there are no other treatment options or the benefits of therapy outweigh its risks. People at high risk include people with a history of blockage or aneurysm of the aorta or other blood vessels or a tendency to do so (for example, with peripheral vascular atherosclerosis), high blood pressure, certain genetic disorders associated with changes in blood vessels (including Marfan syndrome and Ehlers syndrome - Danlos), as well as in old age.
Patients should seek emergency care immediately if there is sudden, severe, or prolonged pain in the abdomen, chest, or back. It is important to understand that symptoms of an aortic aneurysm often do not appear until it grows large or ruptures.
Reports of unsafe use of fluoroquinolones available to the FDA and data from four observational clinical studies have established the risk of aortic aneurysm associated with the use of fluoroquinolones ranging from 9 cases per 100 thousand people per year (general population) to 300 cases per 100 thousand people per year (in the high-risk group). In any case, fluoroquinolones approximately double the risk of aortic aneurysm rupture or aortic dissection.
The images are for illustrative purposes and refer to drugs available on the Russian market. Image sources are in the public domain.
"Mosmedpreparaty"
Fluoroquinolone medicinal compounds, used in medical practice for over 30 years, have pronounced antimicrobial activity with a wide spectrum of action. They are not considered classical antibiotics (although they are very close to them), since they have no natural analogues. The bactericidal mechanism of action of fluoroquinolones is associated with the eradication of microorganisms by interfering with the replication cycle of their DNA. Fluoroquinolone molecules inhibit bacterial topoisomerase II (DNA gyrase) and topoisomerase IV, thereby inhibiting DNA replication and transcription.
The FDA warning applies to fluoroquinolones approved in the United States and used worldwide:
- delafloxacin;
- ciprofloxacin;
- gemifloxacin;
- levofloxacin;
- moxifloxacin;
- ofloxacin.
However, this does not mean that other fluoroquinolone drugs are free from the safety concerns noted above. The range of fluoroquinolones available on the market is impressive (those marked in italics have been withdrawn from sale in civilized countries due to adverse reactions or obsolescence):
First generation fluoroquinolones
- cinoxacin;
- flumequine;
- nalidixic acid;
- oxolinic acid;
- pipemidic acid;
- pyromidic acid;
- rosoxacin.
Second generation fluoroquinolones
- ciprofloxacin;
- enoxacin;
- fleroxacin;
- lomefloxacin;
- nadifloxacin;
- norfloxacin;
- ofloxacin;
- pefloxacin;
- rufloxacin.
Third generation fluoroquinolones
- balofloxacin;
- grepafloxacin;
- levofloxacin;
- pazufloxacin;
- sparfloxacin;
- temafloxacin;
- tosufloxacin.
Fourth generation fluoroquinolones
- alatrofloxacin;
- besifloxacin;
- delafloxacin;
- finafloxacin;
- garenoxacin;
- gatifloxacin;
- gemifloxacin;
- moxifloxacin;
- ozenoxacin;
- sitafloxacin;
- prulifloxacin;
- trovafloxacin.
Fifth generation fluoroquinolones
- nemonoxacin.
safety concerns :
- July 2008: tendonitis and tendon rupture (usually the Achilles tendon)—especially in people over 60 years of age, those on concomitant steroid therapy, or those who have had a kidney, heart, or lung transplant;
- August 2013: peripheral neuropathy, sometimes irreversible;
- May 2021: the risks associated with potentially permanent disability outweigh the benefits of fluoroquinolones and are therefore not recommended for the treatment of acute sinusitis, acute bronchitis or uncomplicated urinary tract infections;
- July 2021: Fluoroquinolones are strongly discouraged for the treatment of acute bacterial sinusitis (ABS), exacerbation of bacterial chronic bronchitis (ABECB), or uncomplicated urinary tract infections (UTI);
- July 2021: a significant decrease in blood sugar levels (which is very dangerous in diabetes mellitus) and mental disorders (impaired attention, agitation, nervousness, memory impairment, delirium, nightmares, paranoia, hallucinations).
Directions for use and doses
Inside. Once or twice a day. The tablets should be swallowed without chewing and washed down with a sufficient amount of liquid (0.5 to 1 glass).
The drug can be taken before meals or at any time between meals, since food intake does not affect the absorption of the drug (see section "Pharmacokinetics").
The drug should be taken at least 2 hours before or 2 hours after taking drugs containing magnesium and/or aluminum, iron, zinc, or sucralfate (see section “Interaction with other drugs”).
Considering that the bioavailability of levofloxacin when taking Levofloxacin Ecolevid® tablets is 99-100%, if the patient is transferred from intravenous infusion with other levofloxacin drugs, taking Levofloxacin Ecolevid® tablets should be continued at the same dose that was used for intravenous infusion of levofloxacin drugs (see section "Pharmacokinetics").
Skipping one or more doses of the drug
If you accidentally miss a dose of the drug, you should take the next dose as soon as possible and then continue to take Levofloxacin Ecolevid® according to the recommended dosage regimen.