Material and methods
At the city office for extrapyramidal diseases of the nervous system (Voronezh), the effectiveness of treatment of patients with PD with the drug stalevo was assessed.
The study included 47 patients, 21 men and 26 women, with stage 3-4 PD (according to Hoehn and Yahr). The age of the patients ranged from 58 to 76 years, the duration of the disease was 6-18 years.
The patients had clinical signs of motor fluctuations with the presence of dose depletion and on-off phenomena. 7 patients had disturbances in the form of choreiform dyskinesia or painful dystonia, 8, in addition, had “night” symptoms (akinesia, myoclonus, restless legs syndrome, urinary disorders). Patients received two-component levodopa preparations for 3 years or more (levodopa dose from 600 to 1500 mg) in combination with non-ergoline dopamine receptor agonists and/or amantadines.
All patients were prescribed the drug stalevo (100-150 mg) in combination with two-component levodopa preparations (¼-½ tablets of levodopa-carbidopa or levodopa-benserazide 3-4 times a day) taking into account the equivalent daily dose of levodopa. For patients on quadruple levodopa dosing, ¾ to a full tablet of levodopa-carbidopa or levodopa-benserazide was prescribed. In the treatment regimen for patients with nocturnal symptoms, the third dose of Stalevo was prescribed before bedtime. For patients with an increased daily dose and frequency of levodopa administration (more than 5 times a day), the treatment regimen was optimized (no more than 1000 mg of levodopa per day).
The effectiveness of treatment was assessed using the Unified PD Rating Scale (UPDRS), the patient's self-monitoring diary, and a questionnaire of motor function fluctuations. Control examinations were carried out after 1, 4 and 6 weeks of treatment.
Stalevo®
Stalevo® is not recommended for the treatment of drug-induced extrapyramidal reactions.
The drug Stalevo® is prescribed with caution to patients with coronary heart disease, severe forms of diseases of the cardiovascular system or lungs, bronchial asthma, kidney disease or endocrine system, with a history of stomach ulcers or convulsions.
In patients who have suffered a myocardial infarction and have lesions of the atrial node or ventricular arrhythmias, it is necessary to monitor heart function, especially during the selection of initial doses.
All patients taking Stalevo® should be carefully monitored for mental changes, depression with suicidal tendencies, and other significant antisocial reactions. The drug Stalevo® is prescribed with caution to patients with psychosis (including a history of psychosis).
Combined treatment with antipsychotics (dopamine receptor blockers, especially D2 receptor antagonists) should be prescribed with caution. Careful monitoring of the patient is necessary for loss of the antiparkinsonian effect of the drug or worsening of symptoms of Parkinson's disease.
The drug is prescribed with caution to patients with chronic open-angle glaucoma; It is necessary to carefully monitor the patient's intraocular pressure and record all changes in pressure.
Taking Stalevo® may cause orthostatic hypotension. The drug is prescribed with caution to patients taking other medications that can also cause orthostatic hypotension.
When combined with levodopa, entacapone may cause drowsiness and episodic instantaneous sleep onset in patients with Parkinson's disease.
When taking the drug, you must be careful when driving vehicles or working with machinery.
Clinical studies have confirmed an increased incidence of dopaminergic adverse reactions (eg, dyskinesia) in patients treated with entacapone and the dopamine agonists (bromocriptine), selegiline and amandatine, compared with patients receiving placebo with this combination of drugs. It may be necessary to adjust the doses of other antiparkinsonian drugs taken by the patient when prescribing Stalevo® to patients who have not previously received entacapone.
Rarely, rhabdomyolysis occurs in patients with Parkinson's disease associated with dyskinesias or neuroleptic malignant syndrome (NMS). Abrupt dose reductions or sudden discontinuation of levodopa should be monitored, particularly in patients receiving antipsychotic treatment. NMS, rhabdomyolysis and hyperthermia are characterized by motor symptoms (muscle rigidity, myoclonus, tremor), changes in mental status (agitation, confusion, coma), autonomic dysfunction (tachycardia, changes in blood pressure), as well as increased levels of creatine phosphokinase in the blood serum. In some cases, only a few of the above symptoms and signs are observed. Early detection of the disease is essential for effective treatment of NMS.
There are reports of a syndrome similar to neuroleptic malignant syndrome, characterized by muscle rigidity, elevated body temperature, changes in mental status and increased serum creatine phosphokinase, also associated with sudden withdrawal of antiparkinsonian drugs. Of the studies conducted in which entacapone was abruptly discontinued, there were no cases of NMS or rhabdomyolysis associated with withdrawal. Since the introduction of entacapone on the market, isolated cases of the development of NMS have been reported, especially when the dose of entacapone and concomitant dopaminergic drugs is suddenly discontinued or reduced. If it is necessary to change treatment with Stalevo® to levodopa and dopa decarboxylase inhibitors, the change should occur gradually; an increase in the dose of levodopa will likely be required.
If general anesthesia is necessary, Stalevo® can be taken as long as the patient is allowed to take fluids and take oral medications. Resumption of drug therapy at the previously prescribed dosage is possible after general anesthesia as soon as the patient is able to independently take the drug tablets.
During long-term treatment with the drug, it is recommended to periodically monitor the functions of the liver and kidneys, as well as monitor the hematological and cardiovascular systems.
It is recommended to monitor the patient's body weight during diarrhea to prevent excessive weight loss. Prolonged, persistent diarrhea that occurs while taking entacapone may be a sign of colitis. In case of prolonged persistent diarrhea, the drug should be discontinued, appropriate treatment should be prescribed and the cause of diarrhea should be established.
Patients should be closely monitored for the development of impulse control disorders. Patients and those involved in their treatment should be warned about the possible occurrence of behavioral symptoms of impulse control disorders, such as gambling, increased libido, hypersexuality, urge to spend money and buy, gluttony or compulsive hunger. These symptoms may occur during treatment with dopamine agonists and/or other dopaminergic drugs containing levodopa, including Stalevo®. If these symptoms appear, it is recommended to reconsider the treatment regimen.
Patients with progressive anorexia, asthenia and weight loss, especially over a relatively short period of time, require a general medical examination and testing of liver function.
Levodopa and carbidopa may cause a false positive result on urine acetone test strips. In this case, boiling the urine test does not change the resulting reaction. The glucose oxidase method may give a false negative result when testing for glycosuria.
The composition of the drug Stalevo® includes sucrose. Patients with rare hereditary diseases such as galactose intolerance, lactose intolerance or glucose-galactose malabsorption should not take this drug.
results
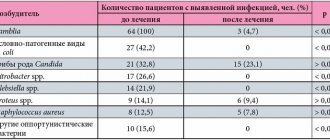
After 6 weeks of treatment, 45 of 47 patients experienced a decrease in the severity or complete disappearance of the dose “depletion” phenomenon, and a decrease in the duration of the “off” phenomenon during the day. In 5 patients the severity and duration of dyskinesia decreased; 2 patients noted a significant decrease in painful cramps and manifestations of restless legs syndrome; 4 patients noted improved sleep and decreased urgency to urinate. In 10 patients, it was possible to reduce the daily dose of levodopa without worsening general symptoms.
When analyzing the observed undesirable side effects, it turned out that 1 patient experienced dyspeptic symptoms in the first 2 days of taking the drug, and 2 patients experienced an increase in dyskinesia, which was stopped by reducing the daily dose of Stalevo. In 3 patients, orthostatic hypotension occurred, which was relieved by correction of antihypertensive therapy.
During treatment, there was a significant decrease in the severity of motor disorders in 42 patients according to part 1 of the UPDRS scale; 33 – in part 2 (everyday activities); in 23 - in the 3rd part (motor disorders) and in 37 - in the 4th part (dyskinesia).
Here is an example of a clinical observation.
Patient X.
, born 1942. In 2006, he consulted a neurologist at his place of residence with complaints of tremors in his right hand, changes in gait (“shuffling with his right foot”), changes in handwriting, and slowness of movements. PD was diagnosed and the drug Nakom was immediately prescribed at a dose of 125 mg 3 times a day. Over the course of 4 years, the patient independently increased the dose of Nakoma to 8 tablets of 250 mg per day. During treatment, the patient developed “twisting” in the limbs, swaying of the head, usually 1-2 hours after taking levodopa, severe trembling (before taking the medicine, and sometimes without connection with taking Nakoma) lasting from 2 to 4 hours, according to several times a day, muscle pain, leg cramps, more often in the evening, frequent urge to urinate, episodes of a sharp drop in blood pressure. The patient was referred for consultation to the office of extrapyramidal diseases of the nervous system.
The examination revealed: tremulous-rigid syndrome, predominantly expressed in the right extremities, motor fluctuations with the phenomena of “depletion” of the dose, “on-off”, drug-induced dyskinesias, postural disorders, the presence of autonomic dysfunction.
Concomitant diseases: coronary heart disease, atrial fibrillation. In 2007, a pacemaker was installed. Hypertension 11A, risk 111. Chronic heart failure. Prostate adenoma. Takes medications: nacom 250 mg 8 tablets, concor, omnic, cordarone, warfarin.
Recommended taking into account concomitant pathology: at 8 o'clock - madopar GSS 1 capsule + stalevo (150 mg) 1 tablet; at 10 a.m. - mirapex PD 0.75 mg; at 12 noon - Stalevo (150 mg) 1 tablet + ½-¼ Nakoma tablets; at 17:00 - ¾ Nakoma tablets; at 21:00 - Stalevo (150 mg) 1 tablet + ½ tablet of Nakoma.
After 1 month, there was a significant improvement in the condition in the form of regression of drug-induced dyskinesias and motor fluctuations, and the severity of autonomic dysfunction decreased. There was good tolerability of the drugs and a lasting therapeutic effect.
Stalevo, 200 mg/200 mg/50 mg, film-coated tablets, 30 pcs.
Stalevo® is not recommended for the treatment of drug-induced extrapyramidal reactions.
The drug Stalevo® is prescribed with caution to patients with coronary heart disease, severe forms of diseases of the cardiovascular system or lungs, bronchial asthma, kidney disease or endocrine system, with a history of stomach ulcers or convulsions.
In patients who have suffered a myocardial infarction and have lesions of the atrial node or ventricular arrhythmias, it is necessary to monitor heart function, especially during the selection of initial doses.
All patients taking Stalevo® should be carefully monitored for mental changes, depression with suicidal tendencies, and other significant antisocial reactions. The drug Stalevo® is prescribed with caution to patients with psychosis (including a history of psychosis).
Combined treatment with antipsychotics (dopamine receptor blockers, especially D2 receptor antagonists) should be prescribed with caution. Careful monitoring of the patient is necessary for loss of the antiparkinsonian effect of the drug or worsening of symptoms of Parkinson's disease.
The drug is prescribed with caution to patients with chronic open-angle glaucoma; It is necessary to carefully monitor the patient's intraocular pressure and record all changes in pressure.
Taking Stalevo® may cause orthostatic hypotension. The drug is prescribed with caution to patients taking other medications that can also cause orthostatic hypotension.
When combined with levodopa, entacapone may cause drowsiness and episodic instantaneous sleep onset in patients with Parkinson's disease.
When taking the drug, you must be careful when driving vehicles or working with machinery.
Clinical studies have confirmed an increased incidence of dopaminergic adverse reactions (eg, dyskinesia) in patients treated with entacapone and the dopamine agonists (bromocriptine), selegiline and amandatine, compared with patients receiving placebo with this combination of drugs. It may be necessary to adjust the doses of other antiparkinsonian drugs taken by the patient when prescribing Stalevo® to patients who have not previously received entacapone.
Rarely, rhabdomyolysis occurs in patients with Parkinson's disease associated with dyskinesias or neuroleptic malignant syndrome (NMS). Abrupt dose reductions or sudden discontinuation of levodopa should be monitored, particularly in patients receiving antipsychotic treatment. NMS, rhabdomyolysis and hyperthermia are characterized by motor symptoms (muscle rigidity, myoclonus, tremor), changes in mental status (agitation, confusion, coma), autonomic dysfunction (tachycardia, changes in blood pressure), as well as increased levels of creatine phosphokinase in the blood serum. In some cases, only a few of the above symptoms and signs are observed. Early detection of the disease is essential for effective treatment of NMS.
There are reports of a syndrome similar to neuroleptic malignant syndrome, characterized by muscle rigidity, elevated body temperature, changes in mental status and increased serum creatine phosphokinase, also associated with sudden withdrawal of antiparkinsonian drugs. Of the studies conducted in which entacapone was abruptly discontinued, there were no cases of NMS or rhabdomyolysis associated with withdrawal. Since the introduction of entacapone on the market, isolated cases of the development of NMS have been reported, especially when the dose of entacapone and concomitant dopaminergic drugs is suddenly discontinued or reduced. If it is necessary to change treatment with Stalevo® to levodopa and dopa decarboxylase inhibitors, the change should occur gradually; an increase in the dose of levodopa will likely be required.
If general anesthesia is necessary, Stalevo® can be taken as long as the patient is allowed to take fluids and take oral medications. Resumption of drug therapy at the previously prescribed dosage is possible after general anesthesia as soon as the patient is able to independently take the drug tablets.
During long-term treatment with the drug, it is recommended to periodically monitor the functions of the liver and kidneys, as well as monitor the hematological and cardiovascular systems.
It is recommended to monitor the patient's body weight during diarrhea to prevent excessive weight loss. Prolonged, persistent diarrhea that occurs while taking entacapone may be a sign of colitis. In case of prolonged persistent diarrhea, the drug should be discontinued, appropriate treatment should be prescribed and the cause of diarrhea should be established.
Patients should be closely monitored for the development of impulse control disorders. Patients and those involved in their treatment should be warned about the possible occurrence of behavioral symptoms of impulse control disorders, such as gambling, increased libido, hypersexuality, urge to spend money and buy, gluttony or compulsive hunger. These symptoms may occur during treatment with dopamine agonists and/or other dopaminergic drugs containing levodopa, including Stalevo®. If these symptoms appear, it is recommended to reconsider the treatment regimen.
Patients with progressive anorexia, asthenia and weight loss, especially over a relatively short period of time, require a general medical examination and testing of liver function.
Levodopa and carbidopa may cause a false positive result on urine acetone test strips. In this case, boiling the urine test does not change the resulting reaction. The glucose oxidase method may give a false negative result when testing for glycosuria.
The composition of the drug Stalevo® includes sucrose. Patients with rare hereditary diseases such as galactose intolerance, lactose intolerance or glucose-galactose malabsorption should not take this drug.
Stalevo
A combined antiparkinsonian drug, which is a combination of levodopa, a metabolic precursor of dopamine, carbidopa, an aromatic amino acid decarboxylase inhibitor, and entacapone, an inhibitor of catechol-O-methyltransferase (COMT).
Levodopa increases dopamine levels in the brain. Dopamine is formed directly from levodopa with the participation of aromatic amino acid decarboxylase. The antiparkinsonian effect of levodopa is due to its conversion to dopamine directly in the central nervous system. Levodopa is quickly decarboxylated in peripheral tissues, turning into dopamine, which, however, does not penetrate the BBB.
Carbidopa inhibits the process of decarboxylation of levodopa and the formation of dopamine in peripheral tissues, which indirectly leads to an increase in the amount of levodopa entering the central nervous system.
As a result of inhibition of dopa decarboxylase, levodopa is biotransformed with the participation of catechol-O-methyltransferase (COMT) into the potentially dangerous metabolite 3-O-methyldopa (3-OMD).
Entacapone is a reversible, specific COMT inhibitor, mainly of peripheral action. Entacapone slows the clearance of levodopa from the bloodstream, which leads to an increase in the bioavailability of levodopa, prolonging its therapeutic effect.
Pharmacokinetics
Levodopa
Suction and distribution
It is quickly, but not completely (20-30% of the dose taken) absorbed from the gastrointestinal tract. Eating foods rich in large amounts of neutral amino acids may delay and reduce absorption.
Cmax after oral administration is achieved within 2-3 hours. Individual bioavailability is 15-33%.
Slightly binds to plasma proteins (10-30%). Vd - 1.6 l/kg.
Metabolism and excretion
Actively metabolized in all tissues by dopa decarboxylase and catechol-O-methyltransferase to dopamine, norepinephrine, epinephrine and 3-O-methyldopa. 75% of the dose taken is excreted in the urine in the form of metabolites within 8 hours. Unchanged is excreted in the urine (35% in 7 hours) and in feces. The total clearance of levodopa is 0.55-1.38 l/kg/h.
T1/2 is 0.6-1.3 hours.
Carbidopa
Suction and distribution
Compared to levodopa, it is absorbed and absorbed somewhat more slowly. Pharmacokinetic data are limited. Approximately 36% bound to plasma proteins.
Individual bioavailability is 40-70%.
Among the metabolites excreted in the urine, the main ones are: alpha-methyl-3-methoxy-4-hydroxyphenylpropionic acid and alpha-methyl-3,4-dihydroxyphenylpropionic acid. T1/2 is 2-3 hours.
Metabolism and excretion
It is biotransformed in the liver to two main metabolites, which are excreted in the urine as glucuronides and unbound structures. Unchanged carbidopa is excreted 30% in the urine. Among the metabolites excreted in the urine, the main ones are alpha-methyl-3-methoxy-4-hydroxyphenylpropionic acid and alpha-methyl-3,4-dihydroxyphenylpropionic acid. T1/2 is 0.6-1.3 hours.
Entacapone
Suction and distribution
Rapidly absorbed from the gastrointestinal tract. Cmax with a single oral dose is achieved after 1 hour. Individual bioavailability is 35% (with a single oral dose of 200 mg).
Binds to plasma proteins by 98%, mainly to albumin; in therapeutic concentrations, it does not displace other drugs with a high degree of complexation (including warfarin, salicylic acid, phenylbutazone, diazepam) from binding with proteins. Vd - 0.27 l/kg.
Metabolism and excretion
Almost completely metabolized. Subject to a first-pass effect through the liver, a small amount of entacapone, which is the (E)-isomer, is converted to the (Z)-isomer (approximately 5% of the total amount of entacapone in blood plasma). The main route of metabolism of entacapone and its active metabolite is conjugation with glucuronic acid.
It is excreted in urine by 10-20% and in feces and bile by 80-90%. The total clearance is about 0.7 l/kg/h. T1/2 is 0.4-0.7 hours.
Due to the short T1/2, there is no true accumulation of levodopa or entacapone upon repeated administration.
Pharmacokinetics in special clinical situations
Pharmacokinetic parameters in younger (45-64 years) and older (65-75 years) patients are the same.
The bioavailability of levodopa is significantly higher in women. The bioavailability of carbidopa and entacapone does not depend on the gender of patients.
The metabolism of entacapone is slowed in patients with mild to moderate liver dysfunction (classes A and B on the Child-Pugh scale), which leads to an increase in the concentration of entacapone in the blood plasma both in the absorption and elimination phases.
Impaired renal function does not affect the pharmacokinetics of entacapone. The pharmacokinetics of levodopa and carbidopa have not been studied in patients with impaired renal function.