Glucophage is a hypoglycemic drug designed to relieve the symptoms of type 2 diabetes. The main component of the drug is metformin, whose action is aimed at glycogen synthesis. After taking the drug, the blood sugar level decreases, but not critically, but gradually. “Glucophage” does not stimulate insulin production, like many analogues, and does not have a hypoglycemic effect at normal glucose levels, which makes the medicine safe and effective at the same time.
The drug should be taken only as prescribed by an endocrinologist. Recommended for long-term use with good tolerability and effectiveness.
Action of the drug
Glucophage tablets quickly relieve the symptoms of hyperglycemia. Their mild action allows you to monitor the course of the disease and timely regulate blood glucose parameters. Along with this effect, the drug has a number of other advantages, the main ones of which doctors consider the prevention of heart, vascular, and kidney diseases.
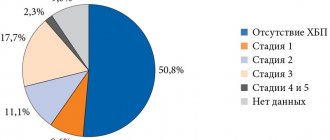
Diabetes mellitus (DM) is an acute medical and social problem that is one of the priorities of the national health care systems of almost all countries of the world.
The first attempts to treat diabetes with oral medications date back to 1926, when Frank, Nothmann and Wagner synthesized and clinically used one of the diguanidine derivatives, synthalin [1].
For several years the drug was used in Germany to treat patients with diabetes, but due to its pronounced hepatotoxic effect it was banned.
In 1959, dimethyl biguanide, metformin, began to be used for the first time. Since the mid-1950s. biguanides began to be widely used in the 1960–1970s. Phenformin and buformin entered medical practice, but their significant drawback was soon noted - the development of lactic acidosis. In 1977, phenformin was banned for clinical use in the USA, Canada and several other countries. In our country it was practically not used.
Glucophage is the original drug of metformin [2]. It has been used as an antihyperglycemic drug for the treatment of type 2 diabetes (T2DM) for more than 50 years. In contrast to sulfonylurea derivatives, which stimulate insulin secretion, Glucophage enhances its effect on peripheral tissues, reducing insulin resistance (mainly in the liver and muscle peripheral tissues) to the action of insulin. The mechanism of action of Glucophage is antihyperglycemic, not hypoglycemic, since the level of glucose in the blood decreases only in cases where it was initially elevated.
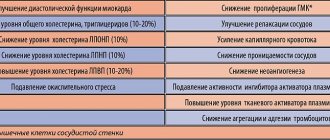
Metformin increases glucose uptake into muscle and adipose tissue by enhancing insulin binding to receptors and increasing the activity of the glucose transporters GLUT-1 and GLUT-4. It reduces the production of glucose by the liver, reduces glycogenolysis and absorption of glucose in the intestine, enhances its utilization, and reduces appetite. In addition, Glucophage improves clinical indicators in patients with both prediabetic conditions (Diabetes Prevention Program) [3] and established T2DM (UK Prospective Diabetes Study) [4]. There is evidence that Glucophage can help reduce the severity of diabetic complications [4].
In recent years, some authors have noted a deterioration in tissue sensitivity to insulin in patients with type 1 diabetes (T1DM). A number of studies indicate the presence of insulin resistance in patients with slowly progressive type 1 diabetes (LADA) [5]. It is noted that with LADA it is higher than with newly diagnosed T1DM, but less than with T2DM. Currently, there are no specific treatment algorithms for patients with LADA. However, many researchers do not recommend sulfonylurea drugs to such patients, since by stimulating insulin secretion in conditions of an autoimmune process, they contribute to its rapid depletion. Taking into account the presence of insulin resistance in LADA, it is considered advisable to use drugs aimed at overcoming it, primarily metformin, which can significantly expand the possibilities of its use [6, 7].
A significant limitation regarding the use of metformin are adverse events (AEs) from the gastrointestinal tract (GIT), which can be observed, according to various authors, in approximately 20% of patients, mainly when initiating therapy [8]. These AEs often disappear with continued use of the drug, as well as with a reduction in its dose. However, it is estimated that 5% of patients completely stop taking metformin due to gastrointestinal AEs. It should also be noted that the pharmacokinetic parameters of metformin make it necessary to take the drug 2-3 times a day. For some patients, this presents certain difficulties, because they are taking several other medications for concomitant diseases. Low compliance with polypharmacy is accompanied by increased mortality [9].
The use of an innovative long-acting form of metformin, Glucophage Long (Glucophage XR in Europe and the USA), may help optimize treatment among many patients receiving a traditional dosage form.
Absorption of standard metformin occurs over a limited area of the upper gastrointestinal tract and is practically absent in its lower parts. The degree of absorption of metformin from the gastrointestinal tract depends on the rate of its evacuation from the stomach.
A new long-acting dosage form of metformin, Glucophage Long, was created using the GelShield diffusion system technology, a feature of which is the presence of a double gel hydrophilic matrix. The outer dense amorphous polymer, which does not contain metformin, surrounds the granules of the inner polymer containing metformin located within it [10].
The unique diffusion system through the GelShield gel barrier provides controlled, sustained release of metformin from the tablet and delayed passage of the tablet itself through the gastrointestinal tract. When swallowed, the Glucophage Long tablet swells and turns into a gel-like mass. Released from the internal polymer, metformin diffuses through the external polymer matrix, entering the gastrointestinal tract for absorption. If, when using the standard form, 90% of metformin is released within 30 minutes, then when treated with Glucophage Long, a similar proportion is released within 10 hours. The rate of release of metformin into the gastrointestinal tract is independent of the severity of peristalsis or changes in pH, which helps to minimize the variability in drug release both within one patient and within the population.
The high efficacy and better tolerability of Glucophage Long have been confirmed in a number of clinical studies involving a large number of patients with T2DM.
The purpose of a double-blind study conducted by S. Schwartz et al. [11], studied the efficacy, tolerability and safety of a new oral extended-release formulation of metformin (Glucophage Long) compared with regular-release metformin when administered to patients with T2DM for 24 weeks.
Adult patients with T2DM (newly diagnosed; receiving non-drug treatment or therapy with oral hypoglycemic drugs) were randomized to one of three long-acting metformin treatment regimens (Glucophage Long 1500 mg/day in 1 dose, 1500 mg/day in 2 doses, 2000 mg/day in 2 doses) or standard metformin (1500 mg/day in 2 doses).
In all groups after 12 weeks, a statistically significant (p < 0.001) decrease in the average HbA1c level was noted. Moreover, in two groups receiving Glucophage Long at 1500 mg/day, the average decrease in HbA1c concentration (-0.73 and -0.74%) did not differ significantly from that in the standard metformin group (-0.70%), while that in patients receiving long-acting metformin therapy at a dose of 2000 mg/day, a more pronounced decrease in HbA1c levels was observed (-1.06%). The rapid decline in fasting plasma glucose levels began in the first week, continued until the eighth week, and was then maintained at this level until the end of the study. Overall, the incidence of AEs was similar in all groups, but fewer patients discontinued treatment early due to initial nausea in the extended-release metformin groups than in the immediate-release metformin group. Thus, therapy with extended-release metformin Glucophage Long 1 or 2 times a day was as safe and effective as therapy with standard metformin 2 times a day, providing a decrease in glycemic levels over 24 weeks of treatment.
K. Fujioka et al. conducted two double-blind randomized clinical trials studying the long-acting metformin Glucophage Long compared with placebo [12]. In one study, 240 patients with T2DM decompensated by diet and exercise were randomized 2:1 to receive long-acting metformin 1000 mg or placebo once daily. For patients with HbA1c levels ≥ 7% and < 8% after 12 weeks of therapy, extended-release metformin (total daily dose of 1500 mg) was prescribed for an additional 12 weeks. The second study was conducted to determine the optimal dose of extended-release metformin Glucophage Long, and included 742 patients with T2DM, decompensated by diet, randomized to placebo or extended-release metformin at doses of 500, 1000, 1500 or 2000 mg once a day or 1000 mg 2 times a day for 16 weeks.
The incidence of diarrhea or nausea/vomiting in these two studies was approximately 50% lower than in another 14-week placebo-controlled study (n = 451) conducted to determine the optimal dose of immediate-release metformin for patients with T2DM. decompensated due to diet [13]. The proportion of patients who prematurely discontinued participation in the study due to gastrointestinal AEs during therapy with Glucophage Long was significantly lower than when using standard metformin (1.8 versus 5.9%). When studying various dosages of extended-release metformin, the dose-dependence of the effects of this form of the drug when taken once a day was shown, with the maximum effect being achieved when using doses of 1500 and 2000 mg/day.
The dynamics of the decrease in HbA1c levels in the group of patients receiving extended-release metformin 1000 mg twice a day (-1.2%) was similar to that in the group of patients receiving 2000 mg of the drug at a time (-1.0%). There was no decrease in efficacy when switching a patient from standard metformin to its extended-release form.
The results obtained confirm the data according to which the glucose-lowering effect of long-acting metformin Glucophage Long when taken once a day is comparable to that of standard metformin prescribed in several doses throughout the day. No clear dose-response relationship has been established for the occurrence of gastrointestinal adverse events when administered extended-release metformin in doses of 1000 to 2000 mg [14].
Subsequently, K. Fujioka et al. conducted a double-blind, randomized study that examined the effectiveness of switching patients with T2DM from conventional metformin to long-acting metformin therapy [15]. Patients received standard metformin 500 mg twice daily for a two-week run-in period, and then 217 were randomized to continue therapy with this drug or switch to the extended-release metformin Glucophage Long at a dose of 1000–1500 mg once daily. The objectives of this study were not to compare drug tolerability, but it was shown that with the use of extended-release metformin, the incidence of gastrointestinal AEs was 10% lower compared to taking a similar dose of standard metformin.
Glucophage Long is available in tablets of 500 and 750 mg, in packs of 30 or 60 pieces. It is recommended to start taking Glucophage Long with one tablet of 500 mg per day, gradually increase the dose to 2 tablets of 750 mg per day with an evening meal. Maximum dosage: 4 tablets of 500 mg or 3 tablets of 750 mg once.
Thus, better tolerability of extended-release metformin is ensured by the original tablet structure. The absorption of metformin from the extended-release tablet (Glucophage Long) is slower and longer than the conventional form, which avoids the occurrence of gastrointestinal adverse events, ensures better tolerability of the drug and helps to increase the adherence of patients with T2DM to metformin therapy.
Indications for use are
- non-insulin-dependent diabetes in adult patients with ineffective diet therapy
- obesity
- type 2 diabetes mellitus in adolescents and children over 10 years of age.
What is the difference between Glucophage and Glucophage Long? Both of these drugs increase the sensitivity of insulin receptors and promote rapid absorption of glucose into muscle tissue. Their action is based on slowing down glucogenesis in the liver and absorption of carbohydrates in the intestines.
Many completely healthy people with diabetes use medications for a completely different purpose: to lose weight. Accelerated lipid metabolism and decreased cholesterol levels in the blood allow you to quickly and effectively lose excess weight.
Glucophage Long has a longer action than its regular counterpart. This allows patients not to divide the daily dose into several doses, but to take only one or two tablets of the drug per day (as prescribed by the doctor). This facilitates the process of treating the disease, creating accessible conditions for busy patients with an active lifestyle.
Otherwise, the effects of the drugs are similar and do not differ in any special ways.
What kind of drug is this
Glucophage is a drug from the biguanide group. The medication is widely used to treat diabetes and eliminate one of the main symptoms of this disease - excess weight. Medicines in this category contain large amounts of metformin. The component has a complex effect on the body and eliminates a significant amount of extra pounds in a short period of time.
Glucophage is produced in the form of white-coated tablets. One package can contain 30, 50, 60 or 100 pieces. The main active ingredient in the drug is metformin hydrochloride. There are several varieties of Glucophage, differing in the concentration of this substance. You can determine the type of drug by adding a certain number to its name - long (500, 700), 850 or 1000. Different types of drugs are used in the treatment of specific stages of diabetes mellitus and differ in their level of effectiveness.
Excipients in the drug, regardless of the concentration of the active component, are:
- magnesium stearate;
- hypromellose;
- povidone;
- microcrystalline cellulose;
- sodium carmellose.
Contraindications for use are also general.
- serious disruption of the functioning of internal organs and systems (diabetic precomas, comas, increased acetone levels)
- renal failure
- severe nervous system disorders
- infectious diseases
- respiratory, heart failure, myocardial infarction
- conditions after abdominal surgery and trauma, when the use of insulin is indicated
- before and after radioisotope and x-ray studies (two days before and after manipulations)
- pregnancy, lactation period
- children under 10 years old
Back Next
Directions for use and dosage
When using Glucophage for weight loss, it is recommended to use only a drug with a concentration of the active ingredient of 500 mg. Other forms of the drug may cause serious harm to health if taken for purposes other than their intended purpose . The maximum weight loss course should be no more than twenty days. You can repeat it only after a few weeks.
Reception regimen:
- You need to take the drug one tablet three times a day;
- The pills should be taken immediately after meals or while eating;
- It is recommended to take the tablets with plenty of water.
Doctors' opinions
Specialists categorically prohibit the use of potent medications unless the patient has indications for their use. Glucophage is no exception. This drug is intended for the treatment of diabetes mellitus and its manifestations. The effect of weight loss in this case is a consequence of the normalization of blood counts and the digestive system. If, despite the warnings of doctors, Glucophage is used for weight loss, then a number of important rules must be followed.
Based on the opinion of experts, the following conclusions can be drawn:
- during the period of taking Glucophage, fasting (even short-term) should be excluded;
- the diet must be complete and balanced (you cannot follow low-calorie diets when taking the drug);
- to obtain maximum results, it is necessary to supplement the diet correction and Glucophage intake with regular physical activity (swimming, morning exercises, cycling, etc.);
- you should absolutely not drink alcohol while taking the drug (in some cases, this combination can cause death);
- Glucophage should not be taken simultaneously with drugs containing iodine and drugs with a diuretic effect;
- If you have any symptoms of intolerance to the drug or a deterioration in the general condition of the body, you should immediately stop taking Glucophage and consult your doctor.