Valtrex, 10 pcs., 500 mg, film-coated tablets
Inside,
regardless of food intake, washed down with water.
Adults
Treatment of herpes zoster
: The recommended dose is 1000 mg 3 times a day for 7 days.
Treatment of infections caused by HSV:
The recommended dose for treatment of an episode is 500 mg 2 times a day for 5 days.
In more severe cases of the onset of the disease, treatment should begin as early as possible, and its duration can be increased from 5 to 10 days. In case of relapse, treatment should continue for 3 or 5 days. For recurrent HSV, it is ideal to prescribe Valtrex® in the prodromal period or immediately after the first symptoms of the disease appear. As an alternative for the treatment of labial herpes, the use of Valtrex® at a dose of 2 g 2 times a day is effective. The second dose should be taken approximately 12 hours (but not earlier than 6 hours) after taking the first dose. When using this dosage regimen, the duration of treatment is 1 day. Therapy should be started when the earliest symptoms of herpes labialis (ie, tingling, itching, burning) appear.
Prevention (suppression) of recurrent infections caused by HSV:
in patients with preserved immunity, the recommended dose is 500 mg 1 time per day. In patients with immunodeficiency, the recommended dose is 500 mg 2 times a day.
Prevention of transmission of genital herpes to a healthy partner:
in infected immunocompetent individuals with relapses no more than 9 times a year, the recommended dose of Valtrex® is 500 mg once a day for a year or more every day.
There are no data on infection prevention in other patient populations.
Adults and teenagers aged 12 years and older
Prevention of CMV infection after transplantation:
The recommended dose is 2 g 4 times a day, prescribed as early as possible after transplantation.
The dose should be reduced depending on creatinine clearance.
The duration of treatment is 90 days, but in high-risk patients the course of treatment can be extended.
Special patient groups
Patients with impaired renal function.
Treatment of herpes zoster and infections caused by HSV, prevention (suppression) of recurrent infections caused by HSV, prevention of transmission of genital herpes to a healthy partner:
the dose of Valtrex® is recommended to be reduced in patients with a significant decrease in renal function (see Table 1). Adequate hydration should be maintained in such patients.
There is no experience with the use of Valtrex® in children with creatinine Cl values less than 50 ml/min/1.73 m2.
Table 1
| Indication | Creatinine Cl, ml/min | Dose of Valtrex® |
| Shingles ( Herpes zoster ) | 15–30 | 1 g 2 times a day |
| less than 15 | ||
| Treatment of infection caused by HSV (according to the regimen of 500 mg 2 times a day) | less than 15 | 500 mg 1 time per day |
| Treatment of labial herpes (according to the regimen of 2 g 2 times a day) | 31–49 | 1 g 2 times a day |
| 15–30 | 500 mg 2 times a day | |
| less than 15 | 500 mg 1 time per day | |
| Prevention (suppression) of recurrent infections caused by HSV | ||
| Patients with normal immunity | less than 15 | 250 mg 1 time per day |
| Patients with reduced immunity | less than 15 | 500 mg 1 time per day |
| Reducing the risk of transmitting genital herpes | less than 15 | 250 mg 1 time per day |
Patients on hemodialysis are recommended to use Valtrex® immediately after the end of the hemodialysis session in the same dose as patients with creatinine Cl less than 15 ml/min.
Prevention of CMV infection after transplantation:
The regimen for prescribing Valtrex® in patients with impaired renal function should be established in accordance with Table 2 below.
table 2
| Creatinine Cl, ml/min | Dose of Valtrex® |
| 75 or more | 2 g 4 times a day |
| from 50 to less than 75 | 1.5 g 4 times a day |
| from 25 to less than 50 | 1.5 g 3 times a day |
| from 10 to less than 25 | 1.5 g 2 times a day |
| less than 10 or dialysis1 | 1.5 g 1 time per day |
1 In patients undergoing hemodialysis, Valtrex® should be prescribed after the end of the hemodialysis session.
It is necessary to frequently determine creatinine clearance, especially during periods when renal function is rapidly changing, for example immediately after transplantation or engraftment, and the dose of Valtrex® is adjusted in accordance with creatinine clearance.
Patients with impaired liver function.
In adult patients with mild or moderate liver dysfunction with preserved synthetic function, no dose adjustment of Valtrex® is required. Pharmacokinetic data in adult patients with severely impaired liver function (decompensated cirrhosis), impaired synthetic liver function and the presence of portacaval anastomoses also do not indicate the need to adjust the dose of Valtrex®, however, clinical experience with this pathology is limited.
Children under 12 years old.
There is no data on the use of Valtrex® in children.
Elderly patients.
No dose adjustment is required unless there is significant impairment of renal function. It is necessary to maintain adequate water and electrolyte balance.
Instructions for use VALTREX™ (VALTREX®)
Treatment of herpes zoster and ophthalmoherpes
Treatment should begin as early as possible, immediately after diagnosing herpes zoster. There are no data available on treatment started more than 72 hours after the onset of rash.
Immunocompetent adults
Valtrex™ is prescribed at a dose of 1000 mg 3 times a day for 7 days (total daily dose is 3000 mg). The dose should be reduced depending on creatinine clearance (see below).
Adults with reduced immunity
Valtrex™ is prescribed at a dose of 1000 mg 3 times a day for at least 7 days (total daily dose is 3000 mg) and for 2 days after crusting. The dose should be reduced depending on creatinine clearance (see below).
Antiviral therapy is recommended for immunocompromised patients who seek medical attention within one week of the blistering period or any time before scabs have completely formed.
Treatment of infections caused by the Herpes simplex virus in adults and adolescents (from 12 years of age)
Immunocompetent adults and adolescents (from 12 years of age)
Valtrex™ is prescribed at a dose of 500 mg 2 times a day (total daily dose is 1000 mg). The dose should be reduced depending on creatinine clearance (see below).
For recurrent episodes, treatment should continue for 3 to 5 days. In primary cases, which may be more severe, the duration of treatment can be increased to 10 days. Treatment should begin as early as possible. For recurrent infections caused by the Herpes simplex virus, it is ideal to prescribe Valtrex™ in the prodromal period or immediately after the first symptoms of the disease appear. Valtrex™ may prevent the development of lesions if therapy is started at the first signs and symptoms of recurrent Herpes simplex infections.
Treatment of labial herpes
For the treatment of labial herpes, the administration of valacyclovir to adults and adolescents at a dose of 2000 mg twice a day is effective. The second dose should be taken approximately 12 hours (but not earlier than 6 hours) after taking the first dose. The dose should be reduced depending on creatinine clearance (see below).
When using this dosing regimen, the duration of treatment should not exceed 1 day, as this excess has not been shown to provide additional clinical benefit. Therapy should be started when the earliest symptoms of labial herpes (tingling, itching, burning) appear.
Adults with reduced immunity
For the treatment of infections caused by the Herpes simplex virus in patients with reduced immunity, Valtrex™ is prescribed at a dose of 1000 mg 2 times a day for at least 5 days after assessing the severity of the clinical condition and the patient’s immune status. In primary cases, which may be more severe, the duration of treatment can be increased to 10 days. Treatment should begin as early as possible. The dose should be reduced depending on creatinine clearance (see below). To achieve maximum clinical effect, treatment should begin within the first 48 hours after the onset of symptoms of the disease. Careful monitoring of damage progression is necessary.
Prevention (suppression) of recurrent infections caused by the Herpes simplex virus in adults and adolescents from 12 years of age
Immunocompetent adults and adolescents from 12 years of age
In immunocompetent patients, Valtrex™ is prescribed at a dose of 500 mg 1 time/day. In patients with very frequent relapses (10 or more per year in the absence of therapy), an additional effect can be achieved by prescribing Valtrex™ at a daily dose of 500 mg, divided into 2 doses (250 mg 2 times / day). The dose should be reduced depending on creatinine clearance (see below). Treatment should be reviewed after 6-12 months of therapy.
Adults with reduced immunity
The recommended dose of Valtrex™ is 500 mg 2 times a day. The dose should be reduced depending on creatinine clearance (see below). Treatment should be reviewed after 6-12 months of therapy.
Prevention of cytomegalovirus infection in adults and adolescents (from 12 years of age)
It is recommended to prescribe Valtrex™ at a dose of 2000 mg 4 times a day as soon as possible after transplantation.
The dose should be reduced depending on creatinine clearance (see below).
The duration of treatment is 90 days, but in high-risk patients treatment may be longer.
Special patient groups
Patients with impaired renal function
Valtrex™ should be used with caution in patients with renal impairment. It is necessary to maintain adequate water and electrolyte balance. It is recommended to reduce the dose of Valtrex™ in patients with impaired renal function (see dose for renal failure in the table).
| Therapeutic indications | Creatinine clearance ( ml/min) | Dose of Valtrex™* |
| Infections caused by the Varicella zoster virus | ||
| Treatment of herpes zoster in immunocompetent and immunocompromised adults | ≥50 30-49 10-29 <10 | 1000 mg 3 times/day 1000 mg 2 times/day 1000 mg 1 time/day 500 mg 1 time/day |
| Infections caused by the Herpes simplex virus | ||
| Treatment of HSV infection: - immunocompetent adults and adolescents - adults with reduced immunity | ≥30 <30 ≥30 <30 | 500 mg 2 times/day 500 mg 1 time/day 1000 mg 2 times/day 1000 mg 1 time/day |
| Treatment of herpes labialis in immunocompetent adults and adolescents (alternative one-day dosing regimen) | ≥50 30–49 10–29 <10 | 2000 mg twice in 1 day 1000 mg twice in 1 day 500 mg twice in 1 day 500 mg once |
| Suppression of HSV infection: - immunocompetent adults and adolescents - adults with reduced immunity | ≥30 <30 ≥30 <30 | 500 mg 1 time/day** 250 mg 1 time/day 500 mg 2 times/day 500 mg 1 time/day |
| Cytomegalovirus infections | ||
| Prevention of cytomegalovirus infection after solid organ transplantation in adults and adolescents | ≥75 from 50 to <75 from 25 to <50 from 10 to <25 <10 or on dialysis | 2000 mg 4 times/day 1500 mg 4 times/day 1500 mg 3 times/day 1500 mg 2 times/day 1500 mg 1 time/day |
* For patients on intermittent hemodialysis, it is recommended to use Valtrex™ after the end of the hemodialysis session on the days of the procedure.
** For the suppression of infections caused by the Herpes simplex virus in immunocompetent patients with a history of at least 10 relapses per year, improved results can be achieved by taking a dose of 250 mg 2 times a day.
In patients on intermittent hemodialysis, Valtrex™ should be prescribed after the end of the hemodialysis session. It is necessary to frequently determine creatinine clearance, especially during periods when renal function changes rapidly, for example, immediately after transplantation or engraftment, and the dose of Valtrex™ is adjusted according to creatinine clearance.
Patients with liver dysfunction
Based on a study using a single dose of valaciclovir 1000 mg in adult patients with mild to moderate liver cirrhosis (with preserved synthetic liver function), no dose adjustment of Valtrex™ is required. Pharmacokinetic data in adult patients with severe liver dysfunction (decompensated cirrhosis), with impaired synthetic liver function and the presence of portacaval anastomoses also do not indicate the need for dose adjustment of the drug Valtrex™, however, the experience of its clinical use in this pathology is limited. For information on the use of higher doses (4000 mg/day or more), see the "Special Instructions" section.
Children
The effectiveness of Valtrex™ in children under 12 years of age has not been evaluated.
Elderly patients
The possibility of renal impairment in elderly patients should be considered and the dose adjusted accordingly (see above). Adequate hydration must be maintained.
Valtrex tablets p/o 500 mg packaging No. 10
Trade name of the drug: Valtrex International nonproprietary name: Valaciclovir Chemical name: 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9yl-methoxy]-ethyl ether, monohydrochloride Dosage form: Film-coated tablets 0.5 g
Description White, film-coated tablet, white or almost white tablet core. Biconvex, oblong, without marks, on one side the inscription GX CF1 is engraved. Composition: Active substance: valacyclovir hydrochloride 556 mg (equivalent to 500 mg valacyclovir). Excipients: microcrystalline cellulose, crospovidone, povidone K 90, magnesium stearate, colloidal anhydrous silicon, white dye concentrate YS-1-18043, carnauba wax. Tablet shell: hydroxypropyl methyl cellulose, titanium dioxide (E171), polyethylene glycol 400, polysorbate 80. Pharmacotherapeutic group: Antiviral agent. ATX Code: [J05AB11]
Pharmacological properties Pharmacodynamics In the human body, valacyclovir is quickly and completely converted into acyclovir under the influence of the enzyme valacyclovir hydrolase. Acyclovir has in vitro specific inhibitory activity against herpes simplex viruses (HSV) types 1 and 2, varicella zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV) and human herpes virus type 6 . Acyclovir inhibits viral DNA synthesis immediately after phosphorylation and conversion to the active form of acyclovir triphosphate. The first stage of phosphorylation requires the activity of virus-specific enzymes. For HSV, VZV and EBV, this enzyme is viral thymidine kinase, which is present in cells affected by the virus. Partial phosphorylation selectivity is conserved in cytomegalovirus and is mediated through the phosphotransferase gene product. Activation of acyclovir by a specific viral enzyme largely explains its selectivity. Phosphorylation process UL 97. Activation of acyclovir (conversion from mono- to triphosphate) is completed by cellular kinases. Acyclovir triphosphate competitively inhibits viral DNA polymerase and, being a nucleoside analogue, is incorporated into viral DNA, which leads to obligate chain breakage, cessation of DNA synthesis and, consequently, blocking viral replication. In immunocompetent patients, HSV and VZV with reduced sensitivity to valacyclovir are extremely rare, but can sometimes be found in patients with severe immunocompromise, for example, with a bone marrow transplant, in those receiving chemotherapy for malignant neoplasms and in HIV-infected patients. Resistance is usually caused by a deficiency of thymidine kinase, which leads to excessive spread of the virus in the host. Sometimes a decrease in sensitivity to acyclovir is due to the emergence of virus strains with a violation of the structure of the viral thymidine kinase or DNA polymerase. The virulence of these variants of the virus resembles that of the wild strain. Clinical studies have shown that valacyclovir reduces the risk of genital herpes infection in a healthy partner when Valtrex is taken as a suppressive therapy in combination with safe sex. Valtrex has been shown to accelerate the resolution of pain, reduce its duration, and reduce the percentage of patients with pain caused by herpes zoster, including acute and postherpetic neuralgia. The effectiveness of Valtrex for the treatment of herpes labialis has also been demonstrated. Clinical studies have demonstrated that prophylactic administration of Valtrex for CMV infection reduces the severity of acute graft rejection (in patients with kidney transplants), opportunistic infections and other herpesvirus infections (HSV, VZV). Pharmacokinetics Valacyclovir is a prodrug of acyclovir. The bioavailability of acyclovir derived from valacyclovir is 3.3 to 5.5 times greater than that observed for oral acyclovir. General information: After oral administration, valacyclovir is well absorbed from the gastrointestinal tract, quickly and almost completely converted to acyclovir and valine. This conversion is catalyzed by the enzyme valacyclovir hydrolase, isolated from the liver after a single dose of valacyclovir at a dose of 250-2000 mg; the average peak concentration of acyclovir in plasma in healthy volunteers with normal renal function is 10-37 μmol (2.2-8.3 μg/ml), and the median time to reach this concentration is 1-2 hours. When taking valacyclovir in a dose of 1000 mg, the bioavailability of acyclovir is 54% and does not depend on food intake. The peak concentration of valacyclovir in plasma is only 4% of the peak concentration of acyclovir, the median time to reach it is 30-100 minutes after dosing, after 3 hours the level of peak concentration remains the same or reduced. Valacyclovir and acyclovir have similar pharmacokinetics. parameters after one-time and repeated use. The degree of binding of valacyclovir to plasma proteins is very low (only 15%). In patients with normal renal function, the plasma half-life of acyclovir after administration of valacyclovir is approximately 3 hours, and in patients with end-stage renal disease, the mean elimination half-life is approximately 14 hours. Valaciclovir is excreted from the body in the urine mainly in the form of acyclovir (more than 80% of the dose) and the acyclovir metabolite 9-carboxymethoxymethylguanine; less than 1% of the drug is eliminated unchanged. Patient characteristics: The pharmacokinetics of valacyclovir and acyclovir are largely unaffected in patients with herpes zoster, herpes simplex virus infections and HIV infection. Studies of the pharmacokinetics of valacyclovir and acyclovir in women during late pregnancy indicate that pregnancy does not affect the pharmacokinetics of valacyclovir. Taking Valtrex at a dose of 1000 mg or 2000 mg does not affect the distribution and pharmacokinetic parameters of valacyclovir in HIV-infected patients compared to healthy individuals. In organ transplant recipients receiving valacyclovir at a dose of 2000 mg 4 times a day, the peak concentration of acyclovir is equal to or greater than that of healthy volunteers receiving the same dose of the drug, and their daily area under the curve is significantly higher.
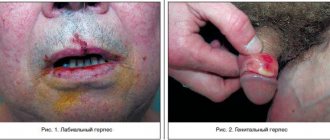
Indications for use Infections caused by the Varicella zoster virus - herpes zoster Treatment of herpes zoster and ophthalmoherpes in adults with preserved immunity, as well as treatment of herpes zoster in adults with mild or moderate immunosuppression. Infections caused by the herpes simplex virus Treatment and suppression of infections of the skin and mucous membranes caused by HSV, including: treatment of newly diagnosed genital herpes in adults and adolescents with preserved immunity and in adults with reduced immunity. treatment of relapses of genital herpes in adults and adolescents with preserved immunity and in adults with reduced immunity. prevention (suppression) of relapses of genital herpes in adults and adolescents with preserved immunity and in adults with reduced immunity. treatment of labial herpes. Treatment and suppression of recurrent ophthalmic infections caused by HSV. Clinical studies have not been conducted in HSV-infected patients with reduced immunity due to causes other than HIV infection. Cytomegalovirus (UMB) infection Prevention of cytomegalovirus (CMV) infection occurring during solid organ transplantation in adults and adolescents.
Contraindications Valtrex is contraindicated in patients with hypersensitivity to valacyclovir, acyclovir and any auxiliary ingredient included in the drug. It should be used with caution in clinically significant forms of HIV infection.
Use during pregnancy and lactation Teratogenicity: Valacyclovir does not have a teratogenic effect in rats and rabbits. Valaciclovir is almost completely metabolized to form acyclovir. Subcutaneous administration of acyclovir did not cause teratogenic effects in rats and rabbits in conventional teratogenicity tests. In additional studies in rats, fetal developmental disorders were identified when the drug was administered subcutaneously in doses that caused an increase in plasma concentrations of acyclovir to 100 mcg/ml and toxic effects in the mother. Fertility: When administered orally, valacyclovir did not cause fertility problems in male or female rats. Pregnancy: There are limited data on the use of valacyclovir and acyclovir during pregnancy from pregnancy registries in women taking valacyclovir or acyclovir (the active metabolite of valacyclovir). Available data and post-marketing data have not shown an increase in the incidence of birth defects in children or feto/neonatal toxicity. Valtrex should be used during pregnancy only if the potential benefit outweighs the potential risk. Lactation: Acyclovir, the main metabolite of valacyclovir, is excreted in the breast. milk. However, when taken by a nursing mother in therapeutic doses, valacyclovir has no effect on breastfed children, since the dose received by the child through breast milk is less than 2% of the therapeutic dose for: intravenous acyclovir for the treatment of neonatal herpes. Valaciclovir should be used with caution during breastfeeding and only when clinically indicated.
Doses and method of administration Treatment of herpes zoster and ophthalmic herpes Treatment should begin as early as possible, immediately after diagnosing herpes zoster. There are no data available on treatment started more than 72 hours after the onset of rash. For adults with preserved immunity, Valtrex is prescribed at a dose of 1000 mg 3 times a day for 7 days. The dose should be reduced depending on creatinine clearance. For adults with reduced immunity, Valtrex is prescribed at a dose of 1000 mg 3 times a day for at least 7 days and for 2 days after crusting. The dose should be reduced depending on creatinine clearance. In immunocompromised patients, antiviral therapy is recommended for one week during the period of blistering or at any time until crusting is complete. Treatment of infections caused by HSV in adults and adolescents (from 12 years of age) For adults and adolescents (from 12 years of age) with preserved immunity, Valtrex is prescribed at a dose of 500 mg 2 times a day. The dose should be reduced depending on creatinine clearance. In case of relapse, treatment should continue for 3 or 5 days. In more severe primary cases, treatment should begin as early as possible, and its duration can be increased from 5 to 10 days. For recurrent HSV, it is considered ideal to prescribe Valtrex in the prodromal period or immediately after the first symptoms of the disease appear. Valtrex can prevent the development of lesions if therapy is started when the first symptoms of HSV relapse appear. Treatment of labial herpes For the treatment of labial herpes (fever on the lips), Valtrex is effectively prescribed to adults and adolescents at a dose of 2 g twice during 1 day: The second dose should be taken after approximately 12 hours (but not earlier than 6 hours) after taking the first dose. The dose should be reduced depending on creatinine clearance. When using this dosing regimen, the duration of treatment should not exceed 1 day, as this excess has not been shown to provide additional clinical benefit. Therapy should be started when the earliest symptoms of labial fever (ie, tingling, itching, burning) appear. Adults with reduced immunity For the treatment of HSV in patients with reduced immunity, Valtrex is prescribed at a dose of 10OO mg 2 times a day for at least 5 days after assessing the severity of the clinical condition and the immunological state of the patient. In more severe primary cases, treatment should be started as early as possible, and its duration can be increased to 10 days. The dose should be reduced depending on creatinine clearance. To achieve maximum clinical effect, treatment should begin within the first 48 hours after the onset of symptoms. Careful monitoring of damage progression is necessary. Prevention (suppression) of recurrent infections caused by HSV in adults and adolescents over 12 years of age Adults and adolescents over 12 years of age with preserved immunity In patients with preserved immunity, Valtrex is prescribed at a dose of 500 mg once a day. In patients with very frequent relapses (10 or more per year), an additional effect can be achieved by prescribing Valtrex at a daily dose of 500 mg, divided into 2 doses (250 mg 2 times a day). The dose should be reduced depending on creatinine clearance. Treatment should be reviewed after 6-12 months of therapy. Adults with reduced immunity For adult patients with immunodeficiency, the recommended dose of Valtrex is 500 mg 2 times a day. The dose should be reduced depending on creatinine clearance. Treatment should be reviewed after 6-12 months of therapy. Prevention of CMV infection in adults and adolescents (from 12 years of age) It is recommended to prescribe Valtrex at a dose of 2 g 4 times a day, as early as possible after transplantation. The dose should be reduced depending on creatinine clearance. The duration of treatment is 90 days, but in high-risk patients treatment may be longer. Doses for renal failure Treatment of herpes zoster, treatment and suppression of infections caused by HSV: Valtrex should be used with caution in patients with renal failure. It is necessary to maintain adequate water and electrolyte balance. It is recommended to reduce the dose of Valtrex in patients with a significant decrease in renal function (see the most detailed instructions included in the package). In patients undergoing hemodialysis, Valtrex should be prescribed after the end of the hemodialysis session. It is necessary to frequently determine creatinine clearance, especially during periods when renal function changes rapidly, for example, immediately after transplantation or engraftment, and the dose of Valtrex is adjusted in accordance with creatinine clearance. Dose of Valtrex for liver dysfunction Studies using valacyclovir at a dose of 1000 mg in adults have shown that in patients with mild to moderate liver cirrhosis (synthetic liver function is preserved), no dose adjustment of Valtrex is required. Pharmacokinetic data in patients with severe liver cirrhosis (with impaired synthetic function of the liver and the presence of shunts between the portal system and the general vascular bed) also do not indicate the need to adjust the doses of Valtrex, however, the experience of its clinical use in this pathology is limited. Doses in children There is no data on the use of Valtrex in children under 12 years of age. Doses in the elderly The possibility of renal impairment in elderly patients should be considered and the dose adjusted accordingly. It is necessary to maintain adequate water and electrolyte balance.
Side effects Adverse reactions are listed below in accordance with the classification by main systems and organs and by frequency of occurrence. Frequencies used: Very common: >1 in 10 Common: >1 in 100 and <1 in 10 Uncommon: >1 in 1000 and <1 in 100 Rare: >1 in 10,000 and <1 in 1000 Very rare: <1 in 10000. Nervous system disorders Very common: headache. Gastrointestinal disorders Common: nausea. Data from post-marketing studies Blood and lymphatic system disorders Uncommon: leukopenia, thrombocytopenia. Basically, leukopenia was observed in patients with reduced immunity. Immune system disorders Rare: anaphylaxis. Mental and nervous system disorders Common: dizziness. Uncommon: confusion, hallucinations, decreased mental capacity, agitation, tremor. Rare: ataxia, dysarthria, psychotic symptoms, seizures, encephalopathy, coma, delirium. Neurological disturbances, sometimes severe, may be associated with encephalopathy and include confusion, agitation, seizures, hallucinations, and coma. These symptoms are usually reversible and are usually observed in patients with impaired renal function or other predisposing conditions. In organ transplant patients receiving high doses (8 g per day) of Valtrex to prevent CMV infection, neurological reactions develop more often than when taking lower doses. Respiratory, thoracic and mediastinal disorders Uncommon: dyspnea. Gastrointestinal disorders Common: vomiting, diarrhea. Uncommon: abdominal discomfort. Liver and biliary tract disorders Uncommon: reversible abnormalities in liver function tests (bilirubin, liver enzymes, etc.), which are sometimes regarded as manifestations of hepatitis. Skin and subcutaneous tissue disorders Common: rashes, including photosensitivity, itching Uncommon: urticaria. Renal and urinary tract disorders. Uncommon: pain in the kidney area, hematuria (often associated with other renal disorders). Rarely: impaired renal function, acute renal failure (especially in elderly patients or in patients with impaired renal function receiving doses of the drug higher than recommended). Renal colic may be associated with impaired renal function. There have been reports of intertubular deposition of acyclovir crystals in the kidneys. Adequate fluid replacement must be ensured. Others: In patients with severe immunocompromise, especially in patients with advanced AIDS, cases of renal failure, microangiopathic hemolytic anemia and thrombocytopenia (sometimes in combination) have been observed in patients receiving high doses of valacyclovir (8 g daily) for long periods of time. Similar complications have been noted in patients with the same diseases but not receiving valacyclovir.
Overdose Symptoms and Signs: Acute renal failure and neurological symptoms, including confusion, hallucinations, agitation, decreased mental capacity and coma, have been observed in patients receiving valacyclovir doses higher than recommended. In addition, adverse reactions such as nausea and vomiting may occur. Caution should be exercised to avoid accidental overdose. Many reports of overdose have involved patients with renal failure and elderly patients receiving repeated doses of valacyclovir because doses were not reduced accordingly. Treatment: Patients should be closely monitored for signs of toxicity. Hemodialysis significantly enhances the removal of acyclovir from the blood and can be considered the method of choice in the management of patients with an overdose of Valtrex.
Interaction with other drugs Caution must be exercised when combining valacyclovir with nephrotoxic drugs, especially in patients with impaired renal function, and regular monitoring of renal function is recommended. This provision applies to the co-prescription of valacyclovir with aminoglycosides, platinum compounds, iodinated contrast agents, methotrexate, pentamidine, foscarnet, cyclosporine, tacrolimus. Acyclovir is excreted mainly unchanged in the urine through active secretion. After taking 1000 mg of valacyclovir, cimetidine and probenecid reduce renal function.
Valtrex®
Suction
After oral administration, valacyclovir is well absorbed from the gastrointestinal tract, quickly and almost completely converted to acyclovir and valine. This conversion is probably carried out by the liver enzyme valacyclovir hydrolase.
When taking valacyclovir in a dose of 1000 mg, the bioavailability of acyclovir is 54% and is not reduced by food intake. The pharmacokinetics of valacyclovir is not dose-dependent. The rate and extent of absorption decrease with increasing dose, resulting in a less proportionate increase in maximum plasma concentration (Cmax) compared to the therapeutic dose range and decreased bioavailability at doses above 500 mg.
Table 1. Results of assessing the pharmacokinetics of acyclovir when taking single doses of valacyclovir from 250 mg to 2000 mg in healthy volunteers with normal liver function
| Pharmacokinetic parameters of acyclovir | 250 mg (N=15) | 500 mg (N=15) | 1000 mi (N=15) | 2000 mg (N=8) | |
| Сmax | µmol/l | 9,78 ± 1,71 | 15,0 ± 4,23 | 23,1 ± 8,53 | 36,9 ± 6,36 |
| µg/l | 2,20 ± 0,38 | 3,37 ± 0,95 | 5,20 ± 1,92 | 8,30 ± 1,43 | |
| Тmax | hours (h) | 0,75 | 1,0 | 2,0 | 2,0 |
| (0,75 — 1,5) | (0,75- 2,5) | (0,75 — 3,0) | (1,5 — 3,0) | ||
| AUC | hµmol/l | 24,4 ± 3,65 | 49,3 ± 7,77 | 83,9 ± 20,1 | 131 ± 28,3 |
| hµg/ml | 5,50 ± 0,82 | 11,1 ± 1,75 | 18,9 ± 4,51 | 29,5 ± 6,36 | |
Cmax - maximum concentration in blood plasma;
Tmax is the time until the maximum concentration in the blood plasma is reached;
AUC is the area under the concentration-time pharmacokinetic curve. Cmax and AUC values reflect the average standard deviation.
Values for Tmax reflect the median value and range of values.
The maximum concentration of valacyclovir in blood plasma is only 4% of the concentration of acyclovir, the median time to reach it ranges from 30 to 100 minutes after taking the drug. 3 hours after dosing, the concentration of valacyclovir reaches the level of quantitative determination or below. Valacyclovir and acyclovir have similar pharmacokinetic parameters after single and multiple doses. VZV and HSV do not significantly alter the pharmacokinetics of valacyclovir and acyclovir after oral administration of valacyclovir.
Distribution
The degree of binding of valacyclovir to plasma proteins is very low (15%). The extent of penetration into the cerebrospinal fluid (CSF) is defined as the ratio of AUC in CSF to AUC in plasma and is about 25% for acyclovir and the metabolite 8-hydroxyacyclovir (8-OH-ACV); about 2.5% for the metabolite 9-(carboxymethoxy)methyl-guanine (CMMG).
Metabolism
After oral administration, valacyclovir is converted to acyclovir and L-valine through first-pass metabolism in the intestine and/or hepatic metabolism. Acyclovir is converted into small metabolites: CMMG under the influence of ethyl alcohol and aldehyde dehydrogenase; 8-OH-ACV under the influence of aldehyde oxidase. Approximately 88% of the total cumulative plasma exposure is due to acyclovir, 11% to CMMG, and 1% to 8-OH-ACV. Valacyclovir and acyclovir are not metabolized by isoenzymes of the cytochrome P450 system.
Removal
In patients with normal renal function, the half-life of acyclovir from the blood plasma after a single or multiple doses of valacyclovir is about 3 hours. Less than 1% of the administered dose of valacyclovir is excreted unchanged by the kidneys. Valaciclovir is excreted from the body by the kidneys mainly in the form of acyclovir (more than 80% of the dose taken) and the metabolite of acyclovir - CMMG.
Special patient groups
Patients with impaired renal function
The elimination of acyclovir correlates with renal function, and acyclovir exposure increases with the severity of renal failure. In patients with end-stage renal disease, the mean half-life of acyclovir following valacyclovir use is approximately 14 hours, compared with approximately 3 hours in those with normal renal function.
Exposures of acyclovir and its metabolites CMMG and 8-OH-ACV in plasma and CSF were assessed at steady state after multiple doses of valacyclovir in 6 patients with normal renal function (mean creatinine clearance 111 ml/min, range 91-144 ml/min), receiving 2000 mg every 6 hours, and 3 patients with severe renal impairment (mean creatinine clearance 26 ml/min, range 17-31 ml/min) receiving 1500 mg every 12 hours.
In severe renal failure, compared with normal renal function, plasma and CSF concentrations of acyclovir, CMMG, and 8-OH-ACV were 2-, 4-, and 5-6-fold higher, respectively. There was no difference in the extent of acyclovir CSF penetration (defined as the ratio of CSF AUC to plasma AUC), CMMG, or 8-OH-ACV between the two populations with severe renal impairment and normal renal function.
Patients with liver dysfunction
Pharmacokinetic data show that in patients with hepatic impairment, the rate of conversion of valacyclovir to acyclovir is reduced, but not the extent of this conversion. The half-life of acyclovir is independent of liver function.
Pregnancy
A study of the pharmacokinetics of valacyclovir and acyclovir in late pregnancy found an increase in the daily AUC value at steady state when taking valacyclovir daily at a dose of 1000 mg per day, which was approximately 2 times higher than the AUC when taking oral acyclovir at a dose of 1200 mg per day.
HIV infection
In patients with HIV infection, the distribution and pharmacokinetic characteristics of acyclovir after oral administration of one or more doses of 1000 mg or 2000 mg of valacyclovir remain unchanged compared with healthy volunteers.
Organ transplantation
The maximum concentration of acyclovir in organ transplant patients receiving 2000 mg valacyclovir 4 times daily was comparable to or higher than the maximum concentration observed in healthy volunteers receiving the same dose. The established daily AUC values can be characterized as noticeably higher.