Introduction
Arterial hypertension (AH) is the most common chronic disease of the circulatory system, the prevalence of which is about 40% among the adult population of the Russian Federation, i.e.
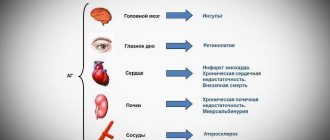
more than 42 million people. Currently, according to the recommendations of the All-Russian Scientific Society of Cardiologists, in the classification of hypertension it is customary to distinguish stages of the process, gradation of hypertension according to the level of blood pressure (BP), as well as stratification of the risk of cardiovascular complications, including identification of risk factors, target organ damage and associated clinical states. Risk factors include male gender, age, smoking, family history of early cardiovascular disease, dyslipidemia, abdominal obesity, impaired glucose tolerance, physical inactivity, and elevated levels of fibrinogen and C-reactive protein. Signs of target organ damage include left ventricular hypertrophy according to ECG (electrocardiographic) criteria and echocardiography (EchoCG), ultrasound signs of arterial wall thickening or atherosclerotic plaques of the great vessels, increased serum creatinine levels, microalbuminuria. Associated clinical conditions include:
- Cerebrovascular diseases: ischemic cerebral stroke, hemorrhagic cerebral stroke, transient ischemic attack.
- Heart diseases: myocardial infarction, angina pectoris, coronary revascularization, chronic heart failure.
- Kidney damage: diabetic nephropathy, renal failure: serum creatinine > 133 µmol/l (1.5 mg/dl) for men or > 124 µmol/l (1.4 mg/dl) for women, proteinuria > 300 mg/day.
- Peripheral artery disease: dissecting aortic aneurysm, symptomatic peripheral arterial disease.
- Hypertensive retinopathy: hemorrhages or exudates, swelling of the optic nerve nipple.
Diabetes mellitus is included in a separate category of factors influencing prognosis. Currently, in terms of the risk of developing cardiovascular complications, it is equal to coronary heart disease and therefore ranks in importance along with associated clinical conditions. Depending on the degree of increase in blood pressure, the presence of risk factors, target organ damage and associated clinical conditions, all patients can be classified into one of four risk levels: low, moderate, high and very high. The risk level is assessed using the SCORE (Systematic COronary Risk Evaluation) system, which estimates the risk of death from diseases associated with atherosclerosis within 10 years. According to this model, a low risk corresponds to a value of less than 4%, a moderate risk – 4–5%, a high risk – 5–8% and a very high risk – more than 8%. Such differentiation into risk groups is important for choosing tactics for managing patients with hypertension. The main goal of treatment of patients with hypertension is to minimize the risk of developing cardiovascular complications and death from them. The target level for the treatment of hypertension is taken to be a blood pressure of less than 140/90 mmHg. Currently, seven classes of antihypertensive drugs are recommended for the treatment of hypertension: diuretics; β-blockers; calcium channel blockers; angiotensin-converting enzyme inhibitors; angiotensin receptor blockers (AT1); imidazoline receptor antagonists; α-blockers. Currently, it is possible to use two strategies for initial treatment of hypertension: monotherapy and low-dose combination therapy. The most commonly used drugs in the treatment of patients with hypertension are angiotensin-converting enzyme and AT1 inhibitors. Numerous multicenter studies have proven their high effectiveness and good tolerability by patients of any age, gender, and with any type of hemodynamics. Drugs in this group combine well with other antihypertensive drugs, as well as with lipid-lowering drugs. AT1 blocks the action of angiotensin II, thereby eliminating pathological vasoconstriction, weakening sympathetic activation, inhibiting interstitial growth in the myocardium and proliferation of smooth muscle cells. In addition, sartans reduce sodium and water retention.
One of the representatives of the new generation of AT1 is azilsartan medoxomil, a specific antagonist of angiotensin II type 1 receptors (AT1). Azilsartan medoxomil is an oral prodrug. It is rapidly converted to the active molecule azilsartan, which selectively interferes with the effects of angiotensin II by blocking its binding to AT1 receptors in various tissues. Angiotensin II is the primary vasoactive hormone of the renin-angiotensin-aldosterone system with effects including vasoconstriction, cardiac stimulation, stimulation of aldosterone synthesis, release and subsequent renal sodium reabsorption.
AT1 receptor blockade inhibits the negative regulatory response of angiotensin II to renin secretion, but the resulting increase in plasma renin activity and circulating angiotensin II levels does not suppress the hypotensive effect of azilsartan.
The hypotensive effect of azilsartan medoxomil develops during the first 2 weeks of use, achieving the maximum therapeutic effect after 4 weeks. A reduction in blood pressure after oral administration of a single dose is usually achieved within a few hours and persists for 24 hours.
The purpose of this study was to study the clinical efficacy and tolerability of azilsartan medoxomil monotherapy in patients with hypertension. The objectives of the study were to conduct an open clinical study of the use of azilsartan medoxomil in patients meeting the criteria for the diagnosis of hypertension of I–II degrees, to evaluate the dynamics of morphofunctional, biochemical and hemodynamic parameters during monotherapy with azilsartan medoxomil, to evaluate the effect of azilsartan medoxomil on the quality of life of patients and the level of anxiety on the scale Hamilton.
Material and methods
The study, which was conducted at the Diagnostic Center No. 5 in Moscow, included 120 patients with grades I–II hypertension. Among them there were 56 (46.6%) men and 64 (53.4%) women. The average age of the group was 49.7±10.04 years. The average duration of hypertension was 7.47±5.65 years. 34 (28.3%) patients suffered from coronary heart disease, and 11 (9.2%) patients suffered from diabetes mellitus.
The duration of the study was 12 weeks.
Inclusion criteria: patient age from 30 to 65 years, blood pressure level at the time of inclusion more than 140/90 and less than 180/110 mmHg, duration of hypertension disease - at least 12 months, signed informed consent of the patients.
Exclusion criteria: symptomatic hypertension, cerebrovascular accident or acute coronary syndrome within the next year, the presence of chronic heart failure of functional classes III–IV according to NYHA (New York Heart Association), decompensated diabetes mellitus type 2 or 1, atrioventricular blockade of II–III degrees , impaired renal and liver function, chronic obstructive pulmonary diseases, pregnancy and lactation, hypersensitivity to the drug or its components.
Patients took azilsartan medoxomil for 12 weeks once a day at an initial dose of 40 mg, followed by a possible increase in case of failure to achieve the target blood pressure level (less than 140/90 mmHg) to 80 mg/day in one dose.
The safety assessment of the use of azilsartan medoxomil was carried out on the basis of data on the side effects of the drug identified during use, taking into account the study of subjective and objective criteria. Depending on the presence and severity of side effects, a conclusion was made about the tolerability of the drug.
During the study (12 weeks), four visits were planned: visit 1 – inclusion (the patient did not receive antihypertensive therapy for 5 days before the inclusion visit). During follow-up visits (2, 3, 4), a physical examination was performed, including determination of body mass index (BMI), waist/hip index (W/H), measurement of blood pressure in sitting and standing positions, and determination of heart rate (HR). ). In addition, laboratory tests were carried out at visits 1 and 4: complete blood count, general urine test, lipid profile, levels of blood glucose, blood potassium, blood creatinine, microalbuminuria were determined, according to a rapid test. Functional studies included ECG (heart rate, voltage criteria for left ventricular myocardial hypertrophy - LV), precise blood pressure monitoring - ABPM (average daily, day and night blood pressure figures, load indices, daily index, blood pressure variability), echocardiography (sizes of heart chambers, hypertrophy LV myocardium). Volumetric sphygmography was performed using a VaSera-1000 device, Japan, with the help of which the brachial-ankle (L-PWV m/s, R-PWV m/s) and cardiobrachial pulse wave velocity (B-PWV m/s) were assessed; pulse wave velocity in the aorta (PWV m/s); mixed arterial (CAVI-1) and aortic (CAVI-2) stiffness indices. During the visits, the level of anxiety on the Hamilton Anxiety Rating Scale (HARS) and the quality of life indicator on the visual analogue scale (VAS) were also determined, and adherence to therapy was assessed.
Statistical processing of the results was performed using the statistical computer program Statistica 6.0. To evaluate the characteristics, average values and standard deviations were calculated; To compare two groups according to one characteristic characterized by a normal distribution, Student's t-test was used. To compare two groups based on a characteristic whose distribution differed from normal, the Mann–Whitney test was used.
Research results
As can be seen from table. 1, the values of BMI, WC/BV, heart rate, lipid metabolism indicators, blood potassium and creatinine levels did not have statistically significant dynamics, however, there was a persistent tendency for these parameters to improve. The dynamics of blood pressure parameters during manual measurement, as well as blood glucose levels, on the contrary, were reliable.
From the table Figure 2 shows that after 3 months of monotherapy with azilsartan medoxomil, changes in ECG and EchoCG parameters were not statistically significant, which should be explained by the short duration of the study. The positive dynamics of all volumetric sphygmography indicators (L-PWV, R-PWV, B-PWV, PWV, CAVI-1, CAVI-2) was significant, which indicates a positive structural and functional restructuring of the vascular wall of elastic and mixed type arteries. The positive dynamics of the following indicators of daily blood pressure monitoring were statistically significant: average daily, day and night blood pressure figures, SBP and DBP load indices, SBP variability.
In table Figure 3 demonstrates statistically significant dynamics in microalbuminuria (MAU) indicators, as well as the level of anxiety and quality of life.
During the study, 21 (17.5%) patients experienced side effects characteristic of taking sartans: increased fatigue, general weakness. All phenomena were short-term and did not require discontinuation of the drug.
Conclusion
According to the results of the study, it was shown that azilsartan medoxomil, used as monotherapy for 3 months in doses of 40 and 80 mg in patients with stage I and II hypertension, is an effective antihypertensive agent that statistically significantly improves blood pressure and ABPM levels. It significantly improves the stiffness of elastic and mixed arteries, according to volumetric sphygmography (VaSera), which helps reduce the rigidity of the vascular wall, prevents vascular remodeling and the development of atherosclerotic changes. The drug is well tolerated by patients. Azilsartan medoxomil is a metabolically neutral drug, and the study revealed a statistically significant decrease in blood glucose levels. Azilsartan medoxomil has been shown to have nephroprotective properties, significantly reducing microalbuminuria. As a result, azilsartan medoxomil significantly improves the quality of life of patients and reduces the level of anxiety on the Hamilton scale.
Edarbi Clo tablets, film coated 40 mg + 25 mg 28 pcs. in Moscow
Inside,
1 time per day, regardless of meal time.
The recommended initial dose of Edarbi® Clo is 40 mg azilsartan medoxomil + 12.5 mg chlorthalidone once a day. If it is necessary to further reduce blood pressure, the dose of Edarbi® Clo can be increased to the maximum - 40 mg of azilsartan medoxomil + 25 mg of chlorthalidone once a day.
Duration of treatment.
Edarbi® Clo should be taken daily, without interruption. If treatment is stopped, the patient must inform the doctor.
Special groups of patients Elderly patients (65 years and older).
No adjustment of the initial dose of Edarbi® Clo is required in elderly patients.
Renal dysfunction.
There is no clinical experience with the use of Edarbi® Clo in patients with arterial hypertension (AH) with severe renal impairment (creatinine Cl less than 30 ml/min), therefore the use of the drug in this category of patients is not recommended (see “Contraindications”).
No dosage adjustment is required in patients with mild to moderate renal impairment (creatinine clearance more than 30 ml/min). Liver dysfunction.
The use of the drug in patients with severe liver dysfunction is not recommended due to the lack of clinical experience with its use (see “Contraindications”).
Due to limited experience with use, Edarbi® Clo should be used with caution in patients with mild to moderate liver dysfunction (less than 9 points on the Child-Pugh scale), since even minor disturbances in water and electrolyte balance when taking diuretics can provoke hepatic coma. It is recommended to actively monitor the condition of such patients. Decrease in BCC.
It is necessary to replenish fluid and electrolyte losses in patients with reduced blood volume before starting the use of Edarbi® Clo (see “Special Instructions”).
Heart failure.
Due to the lack of clinical experience, Edarbi® Clo should be used with caution in patients with hypertension and severe CHF (functional class IV according to the
NYHA
).
Negroid race.
No dose adjustment is required, because The antihypertensive effect of the drug Edarbi® Clo in patients of the Negroid race is similar to its effect in patients of other races.
Missing a dose
If a dose is missed, the patient should take the next dose at the usual time. You should not take a double dose of Edarbi® Clo. Withdrawal syndrome (a sharp increase in blood pressure after sudden withdrawal) was not observed after long-term therapy (for 6 months) with azilsartan medoxomil. However, discontinuation of Edarbi® Clo after long-term treatment should be carried out, if possible, gradually.