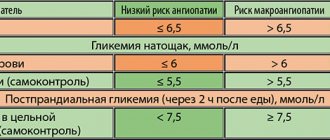
Until the 20th century, sulfonylurea derivatives (SUM) and biguanides (metformin) were used in the treatment of type 2 diabetes mellitus (T2DM); their mechanism of action remained unclear for a long time [1]. The discovery in the 80–90s of the characteristics of incretin degradation and their effect on insulin secretion in humans contributed to the search for drugs that affect this link in the regulation of carbohydrate metabolism. In 1998, the first dipeptidyl peptidase-4 (IDPP-4) inhibitor, vildagliptin, was discovered, named after Edwin B. Willhauer [2, 3], who discovered this molecule. After the successful completion of clinical trials, vildagliptin was first registered in 2007. In Russia, the drug was registered in 2008. Along with other representatives of the STD-4 class, vildagliptin played a key role in changing the paradigm of T2DM treatment from a symptomatic decrease in glucose levels to a pathophysiological approach [4, 5 ].
Pharmacological properties
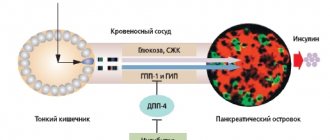
Incretins—glucose-dependent insulinotropic polypeptide (gip) and glucagon-like peptide-1 (gLP-1)—are intestinal hormones involved in carbohydrate homeostasis. They cause insulino- and glucagonotropic effects.
The main effect of these hormones (incretin effect) is to increase glucose-dependent insulin secretion by β-cells (insulinotropic effect) upon food intake. Normally, the contribution of the incretin effect to the postprandial rise in insulin levels is 50–70% [6, 7].
Incretins also have a glucagonotropic effect: they reduce glucagon levels in the postprandial period (glp-1) and maintain or enhance its secretion when glucose concentrations decrease (hyp), preventing the development of hypoglycemia [8, 9].
Already from the stage of impaired glucose tolerance, a decrease in the incretin effect is recorded, which may be an early marker of β-cell dysfunction [10]. Incretin-based therapy has shown to be effective in improving β-cell function [11–13]: the glucose-dependent action of IdPP-4 contributes to the adaptation of β-cells to existing insulin resistance and increasing their sensitivity to glucose [14–17].
An increase in the levels of GLP-1 and Hip is observed 10-15 minutes after a meal [18]. Normally, only ~10% of active incretins reach the systemic circulation, since they are rapidly destroyed by local (~75%) and hepatic DPP-4 (~15%).
Vildagliptin covalently binds to the active site of DPP-4 and blocks its function longer than other drugs (~55 min); the vildagliptin/DPP-4 complex slowly dissociates, and a new drug molecule interacts with the active center of the enzyme. This ensures a prolonged increase in the concentration of incretins [11], due to which the glucose level is stabilized for a long time; in addition to postprandial regulation of the insulin/glucagon ratio, nocturnal gluconeogenesis in the liver and fasting plasma glucose (FPG) levels decrease. By optimizing glucagon secretion, the risk of hypoglycemia is eliminated.
DPP-4 activity in T2DM is associated with oxidative stress, inflammation, and impaired insulin sensitivity [20]. DPP-4 expression in adipose tissue correlates with BMI, amount of visceral fat, adipocyte size, inflammation, and glycated hemoglobin levels [21]. DPP-4, secreted by adipocytes, affects the conduction of the insulin signal in the cells of adipose and muscle tissue [22]. Expression of DPP-4 in the liver correlates with insulin resistance [23]. Recent studies have revealed a correlation of circulating DPP-4 activity with the risk of developing T2DM and atherosclerosis [24, 25].
Thus, in addition to glycemic control, IDPP-4 therapy can also cause extrapancreatic effects: it promotes the mobilization of lipids during meals, reducing the secretion of apolipoprotein b-48 in the intestine, reducing fasting lipolysis and fat content in the liver, as well as increasing the dispersion of LDL and reducing inflammatory response in T2DM.
Metabolic stress in T2DM causes hyperactivation of the immune system, which is manifested by an increase in the level of acute phase markers of inflammation, blood coagulation factors, and proinflammatory cytokines (TNF-α, IL-1β and IL-6). There is also a known connection between insulin resistance and the switching of macrophage phenotype from anti-inflammatory (M2) to pro-inflammatory (M1) [19]. Many studies have shown the anti-inflammatory effects of glucose-lowering drugs, but the effects of reducing glucose toxicity should be distinguished from the effect of drugs on immune components.
GLP-1 receptors and CD26, which have DPP-4 activity, are expressed on the surface of immune cells. IDPP-4 increases the concentration of active GLP-1 and suppresses the activity, signaling and expression of CD26. GLP-1 has an anti-inflammatory effect associated with the reverse switching of macrophages from the M1 to the M2 phenotype, reduces the levels of prostaglandin E2, IL-6 and glycated albumin; due to CD26 inhibition, the levels of C-reactive protein, TNF-α, IL-1β and IL-6, TPR-4, TPR-2 and IkB kinase β decrease [19, 26].
The function of incretins directly depends on the balance of microflora in the intestines. In T2DM, the number of butyrate-producing bacteria, whose metabolites—short-chain fatty acids (SCFAs)—are important regulators of intestinal health, decreases [27]. SCFAs, through receptors (FFAR2 and FFAR3) expressed on enterocytes, support local immunity and intestinal barrier function, reduce endotoxemia and proinflammatory processes associated with T2DM. In addition, SCFAs improve the sensitivity of peripheral tissues to insulin and are involved in the regulation of metabolism and eating behavior reactions [28].
SCFAs promote the production and secretion of GLP-1 and peptide Y. Activation of adipocyte FFAR2 suppresses fat accumulation in adipose tissue and enhances leptin synthesis [28].
An imbalance of the microflora leads to a decrease in the expression of GLP-1 receptors (and neuronal NO synthase) in enteric neurons, which leads to changes in afferent signals of the vagus nerve. Thus, the state of the microbiota influences resistance to GLP-1 and is directly related to the physiological effects realized along the gut-brain axis [29].
There is evidence of the effect of glucose-lowering drugs on the state of the intestinal microbiota [30]. IDPP-4 (specifically vildagliptin) has been shown to increase the number of butyrate-producing bacteria. This is associated with a decrease in edema in the intestinal wall, suppression of parietal inflammatory processes [31] and the activity of DPP-4 microorganisms [32, 33].
Thus, vildagliptin prolongs the action of incretins, regulates glucose homeostasis in a glucose-dependent manner (without hypoglycemia and weight gain) and additionally affects metabolism, inflammation and other parts of the pathogenesis of T2DM [2, 11].
Possibilities and advantages of vildagliptin in combination glucose-lowering therapy
The vast majority of review works devoted to the problem of type 2 diabetes mellitus (T2DM) primarily note that T2DM is a heterogeneous disease. Moreover, the term “heterogeneity” covers not only the pathogenetic basis of T2DM, but also the features of the course of the disease, including the response to therapy. Of course, such heterogeneity is inherent in many other chronic diseases, for example, arterial hypertension, etc. As rightly noted by G. Leibowitz et al. (2009), biological variability is one of the integral manifestations of both life itself and disease.
Rice. 1. Program to study the effectiveness of vildagliptin in type 2 diabetes
For many years, researchers could not answer what is primary in the development of T2DM: insulin resistance or impaired insulin secretion. Only relatively recently has it been concluded that the metabolic abnormalities characteristic of diabetes are the result of a stable combination of both factors. Accordingly, the key to successful treatment of the disease should be an integrated approach that affects all parts of the pathogenesis.
Metformin as a universal first-line drug in the treatment of type 2 diabetes mellitus
In European countries, metformin has been used for the treatment of type 2 diabetes mellitus (T2DM) since the late 50s of the last century. Over the past period of time, numerous clinical studies have confirmed not only the leadership of metformin as an insulin sensitizer, but also discovered a number of unique beneficial effects of the drug on the cardiovascular system, including on the lipid spectrum, blood coagulation factors, etc. [ 24, 27].
According to the latest recommendations of the American and European Diabetes Associations, metformin should be prescribed from the moment of diagnosis of type 2 diabetes mellitus along with lifestyle changes [21]. Thus, metformin is currently the most widely used drug among oral hypoglycemic agents.
The main mechanism of action of metformin is to reduce the production of glucose by the liver, which helps normalize fasting glucose levels. This effect is primarily due to the direct blockade of enzymes involved in gluconeogenesis (phosphoenolpyruvate carboxykinase, fructose-1,6‑biphosphatase and glucose-6‑phosphatase), reducing the uptake by the liver of the starting products necessary for gluconeogenesis (alanine, pyruvate, glycerol and etc.), as well as increased phosphorylation of the insulin receptor and its substrates (SIR-1, SIR-2). On the other hand, metformin enhances the process of glucose utilization in the liver by activating glycolytic enzymes (hexokinase, pyruvate kinase). In addition, the drug suppresses the activity of the liver enzyme acetyl-CoA carboxylase, which helps reduce lipogenesis, enhance the oxidation of free fatty acids (FFA), prevent steatosis and reduce triglyceride synthesis [4, 9, 14, 20, 28].
As it became known relatively recently, the most important mediator of the effect of metformin on gluconeogenesis and lipogenesis is adenosine monophosphate-activated protein kinase (AMPK).
Initially, AMPK was identified only as an enzyme involved in the synthesis of cholesterol and free fatty acids. Research in recent years has identified a much wider range of actions of AMPK: the main function turned out to be the assessment and maintenance of sufficient energy reserves in various cells of the body.
The activity of the enzyme directly depends on the AMP/ATP ratio. Conditions that increase this ratio (energy deficit) and therefore increase AMPK levels include exercise, fasting, hypoxia, ischemia, oxidative stress and heat shock. Activated AMPK helps suppress catabolic processes associated with ATP consumption and stimulates anabolic types of metabolism, ensuring ATP regeneration. Ultimately, the energy reserves in the cell are restored. Thus, AMPK is a kind of fuel sensor (“fuel meter”) in the cells of the body [29].
As the results of fundamental studies have shown, metformin increases the activity of AMPK, which leads to suppression of genes encoding key enzymes of gluconeogenesis and lipogenesis, followed by increased glycogen synthesis in the liver [30].
In skeletal muscle cells, metformin enhances both basal and insulin-stimulated glucose utilization, which, in turn, affects postprandial glycemic values. Activation of AMPK in muscles under the influence of metformin increases glucose utilization by enhancing the translocation of the glucose transporters GLUT4 [12, 30].
In adipose tissue, by activating AMPK, metformin inhibits lipogenesis, enhances glucose utilization and FFA oxidation, and suppresses the secretion of proinflammatory diabetogenic cytokines [10, 12, 22, 16].
Considering that the main cause of death in type 2 diabetes mellitus is cardiovascular diseases, it is extremely important that hypoglycemic drugs have at least a neutral, and, as a priority, a pleiotropic positive effect on the cardiovascular system.
To a certain extent, the renaissance of metformin as a biguanide was justified by the results of the UKPDS study, which for the first time showed that the use of the drug, compared with the use of diet therapy, significantly reduces the risk of all deaths, as well as death from diabetes and a number of other adverse outcomes of the disease (myocardial infarction, cardiac failure, etc.) [25]. Further research made it possible not only to clarify the mechanisms of such effects of metformin, but also, in the words of N. Wiernsperger (2007), to characterize it as a cardiovascular drug with a hypoglycemic effect.
The protective effect of metformin against cardiovascular diseases is due to the direct positive effect of the drug on tissue perfusion, the hemostasis system, oxidative stress, and protein glycation. It is important that this effect occurs when the drug is prescribed in much lower dosages compared to those that determine its glucose-lowering effects [18, 26].
Metformin therapy is characterized by an extremely low risk of side effects (including hypoglycemia and lactic acidosis) and does not increase body weight.
Thus, the evolution of metformin from an ordinary, ancient drug of the pharmacopoeia to a first-line drug in the structure of the most modern regimens for initiating therapy for T2DM is completely justified.
Efficacy of vildagliptin in combination with metformin
The cause of the progressive course of T2DM is primarily the worsening of disorders of the secretory function of pancreatic β-cells; accordingly, in most patients, metformin therapy at a certain stage of the disease requires the addition of drugs that enhance insulin production. The ideal drugs to be combined with metformin are those that have a complementary mechanism of action and provide adequate control of carbohydrate metabolism with minimal risk of additional side effects.
New promising methods for treating T2DM include drugs whose action is associated with enhancing the effects of glucagon-like peptide-1 (GLP-1), a hormone produced by enteroendocrine L-cells of the intestine.
The participation of GLP-1 in the regulation of carbohydrate metabolism is carried out through many mechanisms, the most important of which are:
- increased insulin secretion via a glucose-dependent mechanism;
- suppression of glucagon secretion;
- decreased gastric motility (slow absorption of carbohydrates);
- decreased appetite;
- suppression of β-cell apoptosis, increased proliferation and neogenesis from progenitor cells located in the pancreatic ducts.
The period of residence of the hormone in its active form - GLP-17-36 or GLP-17-37 - is about 2 minutes, then it undergoes rapid destruction with the formation of metabolites GLP-19-36 and GLP-19-37 under the action of the enzyme dipeptidyl peptidase. 4 (DPP-4). Patients with T2DM have lower levels of the hormone than healthy people.
Drugs that inhibit DPP-4 activity (gliptins) increase the lifespan of endogenous glucagon-like peptide-1 [23].
Vildagliptin is a powerful, highly selective DPP-4 inhibitor; within 24 hours it suppresses DPP-4 activity by 97% [1, 3, 19].
Numerous clinical studies involving over 22 thousand patients with T2DM have shown the effectiveness of vildagliptin when used both as monotherapy and in combination with other hypoglycemic agents. The clinical research program to study the efficacy and safety of vildagliptin is schematically presented in Figure 1.
As has become known in recent years, metformin increases GLP-1 levels. Among the proposed mechanisms, the role of biguanide in directly enhancing the secretory function of intestinal L-cells, activating transcription/translation of the proglucagon gene, reducing renal excretion of GLP-1, and blocking dipeptidyl peptidase-4 is discussed [17]. The combination of vildagliptin with metformin has a synergistic effect on enhancing the secretion of GLP-1.
To date, a whole series of studies has been carried out demonstrating the advantages of combination therapy with vildagliptin and metformin.
Vildagliptin in combination with metformin compared with placebo
One of the earliest randomized studies showed that the combination of vildagliptin with metformin can achieve significant improvements in carbohydrate metabolism, and these results remain stable over a year. In this study, vildagliptin at a dose of 50 mg was added to metformin therapy, which after 12 weeks led to a decrease in HbA1c by 0.6% compared to baseline (7.7%). There were no significant changes observed in the control group (metformin monotherapy). Over the next 40 weeks, during the combination therapy, a positive treatment result remained, while in the control group progressive negative dynamics were observed (an increase in HbA1 by 0.066% monthly). Due to this, the difference between groups in HbA1c changed from -0.7% (12th week of observation) to -1.1% (40th week) [2].
In a double-blind study performed by E. Bosi et al. (2007), vildagliptin at a dose of 50 mg once or twice a day was combined with metformin (> 1500 mg per day) in 416 patients. The average HbA1c level, initially 8.4%, increased by 0.2% after 24 weeks with metformin plus placebo. In the group that received, in addition to metformin, vildagliptin at a dose of 50 mg or 100 mg, HbA1c values decreased by 0.5% and 0.9%, respectively, and fasting glycemia levels decreased by 0.8 mmol/l and 1.7 mmol/l. l [8]. It is important that with the combined administration of vildagliptin and metformin, side effects from the gastrointestinal tract were observed less frequently than with metformin monotherapy [11].
Vildagliptin compared with thiazolindiones when added to metformin
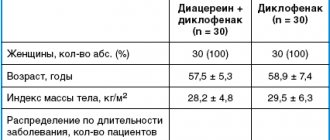
The purpose of the work by G. Boli et al. (2008) assessed the effectiveness and tolerability of vildagliptin compared with pioglitazone in patients with type 2 diabetes with unsatisfactory carbohydrate metabolism (HbA1 within 7.5% - 11%) during metformin monotherapy, used at an average dose of 2000 mg per day. A multicenter, randomized, double-blind study lasting 24 weeks included 295 patients who received, in addition to metformin, vildagliptin 100 mg per day, as well as 281 patients who received pioglitazone 30 mg per day in addition to metformin. By the end of the observation period, the HbA1c level in the group receiving vildagliptin decreased by 0.9%, and in the group receiving pioglitazone - by 1.0% (no statistically significant difference was detected between the groups). Serious side effects occurred in 2% of people on vildagliptin, and in 4.6% on pioglitazone. Moderate hypoglycemia was observed in only one patient (0.3%) receiving vildagliptin. Taking pioglitazone led to a significant increase in body weight (+1.9 kg on average, the difference between the studied groups was 1.6 kg, p
Similar results were obtained in the open multicenter study GALIANT (GALvus In Addition to metformin vs. Tzd/metformin in lowering HbA1c) involving 2664 patients with type 2 diabetes mellitus. Patients received, in addition to metformin at a dose of 1000 mg/day, vildagliptin or thiazolidinediones (the choice of a specific drug - pioglitazone or rosiglitazone - was made by the researchers depending on their experience in practice). After 12 weeks, it was determined that the reduction in HbA1c was similar across groups (-0.68% with vildagliptin vs. -0.57% with thiazolidinediones), and there was no difference in the incidence of side effects overall (respectively, 39.5 % vs. 36.3%). Body weight increased in the group receiving glitazones (+0.33 kg) and decreased in the group receiving vildagliptin (-0.58 kg) [5].
Vildagliptin compared with a sulfonylurea (glimepiride) when added to metformin
The double-blind study included 2789 patients who initially received monotherapy with metformin at an average dose of 1900 mg per day (initial HbA1 with a range of 6.5% - 8.5%), followed by randomization into groups with either vildagliptin 100 mg per day. , or glimepiride up to 6 mg per day. After a year of observation, no difference in the dynamics of HbA1 was detected (-0.4% on vildagliptin vs. -0.5% on glimepiride). Target HbA1 values of up to 7% were achieved by 54% of patients receiving vildagliptin, and 56% of people receiving glimepiride therapy (p>0.05). Despite the similarity of results characterizing the dynamics of carbohydrate metabolism, the number of side effects with vildagliptin was significantly less. Thus, body weight decreased with vildagliptin, but increased in patients receiving glimepiride (the difference between groups was 1.8 kg). Vildagliptin had either a neutral or positive effect on blood lipid parameters; Taking glimepiride was accompanied by a moderate increase in low-density lipoproteins and triglycerides. The incidence of hypoglycaemic episodes at 52 weeks with vildagliptin was 1.7% (no severe cases) and 16.2% with glimepiride (10 severe cases). The clear positive dynamics of carbohydrate metabolism in combination with a low risk of hypoglycemia, even in patients with relatively low HbA1c values, indicates that the hypoglycemic effect of vildagliptin is primarily due to an increase in the sensitivity of the pancreatic secretory apparatus to glucose [13].
Combination vildagliptin/metformin compared with monotherapy with vildagliptin or metformin
In 2009, E. Bosi et al. conducted a 24-week study in which 1179 patients, depending on the nature of therapy, were divided into 4 groups: a combination of vildagliptin 50 mg with metformin 1000 mg twice a day, a combination of vildagliptin 50 mg with metformin 500 mg twice a day, vildagliptin monotherapy (50 mg each). mg twice a day) or monotherapy with metformin 1000 mg twice. The dynamics of HbA1c in the studied groups amounted to -1.8%, respectively; –1.6%; -1.1%; -1.4%. The reduction in fasting blood glucose was also most significant in the groups receiving vildagliptin in combination with metformin compared with patients receiving either drug alone. All treatment regimens were well tolerated by patients and showed similar profiles and rates of side effects. The change in body weight (initial values - on average 88.3 kg) with vildagliptin was -0.6 kg, -1.6 kg with metformin and on average -1.2 kg with both combination therapy regimens [8].
The advantages of combined administration of vildagliptin and metformin (additive effect on carbohydrate metabolism and complementary mechanism of action) predetermined the need to create a fixed combination of these drugs [17]. In the European Union, vildagliptin/metformin (Eucreas) has been used since 2007; in the Russian Federation, the drug Galvus Met was registered in 2009.
Thus, the possibility of simultaneously influencing the key links in the pathogenesis of T2DM - insulin resistance and dysfunction of pancreatic islet cells, a clear improvement in carbohydrate metabolism with a low risk of hypoglycemia - provides a high potential for combination therapy with vildagliptin and metformin.
Efficiency
The effect of vildagliptin on hba1c levels in humans was demonstrated in a 4-week placebo-controlled study in patients with a baseline hba1c level of 7.1–7.2. The effects observed in animal studies were confirmed: an increase in GLP-1 and pancreatic β-cell function, and for the first time a decrease in glucagon levels during meals was established [2].
Initial therapy
Monotherapy with vildagliptin
According to a study involving 354 naïve patients with T2DM, vildagliptin monotherapy for 24 weeks helps reduce glycemia. In those taking vildagliptin in various dosages, ΔHbA1c was 0.4-0.8%, in the placebo group - only 0.1% [34].
Vildagliptin, like metformin, improved glycemic control. In a 52-week study, vildagliptin resulted in a ΔHbA1c of 1% ( p
<0.001), and metformin - 1.4% (
p
<0.001).
When the study was extended to 104 weeks, the results were similar - 1.0 and 1.5%, respectively ( p
<0.001).
There were no differences in the dynamics of the patients’ body weight, the frequency of hypoglycemia was low in both groups ( p
<1%), and adverse events (AEs) from the gastrointestinal tract occurred 2 times more often when taking metformin [35, 36].
In a pooled analysis of the results of vildagliptin monotherapy, it was demonstrated that ΔHbA1c depends on its baseline level. With an initial level of glycated hemoglobin of 10.6%, ΔHbA1c was 2.1%; at 9.5% - 1.8%; at 8.5% - 1.2%; at 7.7% - 0.7% and at the initial HbA1c level of 6.9% - 0.5% [37].
Combination therapy with vildagliptin and metformin
Initial combination therapy with vildagliptin and metformin (2 times daily) was studied in a 24-week clinical trial ( n
=1179, hba1c=7.5—11%). Patients were randomized into combination therapy groups: vildagliptin + metformin at a high dose (50+1000 mg) and low dose (50+500 mg), as well as into monotherapy groups: metformin (1000 mg) and vildagliptin (50 mg). ΔHbA1c in the respective groups was 1.8, 1.6, 1.4 and 1.1% (Fig. 1).
Rice. 1. Changes in HbA1c levels in groups with different T2DM treatments [38]. There were no episodes of hypoglycemia or weight gain in the combination therapy groups. All treatments were well tolerated and showed similar rates of AEs [38].
Intensification of therapy
Dual-component therapy
In a multicenter randomized clinical trial (RCT), vildagliptin 50 mg/day was added to metformin ≥1500 mg/day ( n
=143), vildagliptin 50 mg 2 times a day (
n
=143) or placebo (
n
=130). After 24 weeks, the level of hba1c in the groups of patients receiving vildagliptin decreased by 0.7 and 1.1%, respectively, and hpn by 0.8 and 1.7 mmol/l; in the placebo group, glycemic parameters did not change [39].
When comparing the effects of vildagliptin + metformin and glimepiride + metformin during a 1- and 2-year study [12, 13], the combinations showed similar effectiveness, however, when taking vildagliptin, the number of hypoglycemia was 14 times less (glimepiride: 554 events / 10 severe; vildagliptin: 39 events/0 severe) and no weight gain was noted [40, 41].
Triple therapy
In patients receiving metformin+PSM, the addition of vildagliptin improved glycemic control [42]. After 24 weeks of therapy, glimepiride + metformin with the addition of vildagliptin ( n
=158) ΔHbA1c was 1.01% (
p
<0.001), and in the placebo group (
n
=160) it was 0.25% (
p
<0.001).
In the vildagliptin group, 28.3% of patients reached the target hba1c level, in the placebo group - 5.6% of patients ( p
<0.001); the incidence of hypoglycemia was low and no significant weight gain was observed. The positive effect of adding vildagliptin to metformin + psm is associated not only with an increase in glucose-dependent secretion of β-cells, but also with the normalization of postprandial secretion of glucagon by α-cells.
Combination with insulin
In patients on insulin therapy, vildagliptin improves glycemic control and allows a reduction in the insulin dose [43]. In addition, vildagliptin reduces the incidence of hypoglycemia, which is likely due to increased sensitivity of α-cells to glucose [43, 44].
Efficacy and safety of vildagliptin at a dose of 50 mg twice daily ( n
=144) compared with placebo (
n
=152) were evaluated in patients on insulin-NPH therapy (dose >30 U/day for 4 weeks) with unsatisfactory glycemic control (hba1c 7.5-11%). In the vildagliptin group, ΔHbA1c was 0.5%, in the placebo group - 0.2%, and in patients over 65 years of age - 0.7 and 0.1%, respectively. Vildagliptin reduced the risk of hypoglycemia compared with placebo: 113 (0 severe) versus 185 events (6 severe), respectively [43].
In a 12-week prospective multicenter study, the addition of vildagliptin 50 mg/day reduced the mean daily insulin dose from 36.26 ± 18.21 to 26.87 ± 16.49 U ( p
<0,0001) [44].
Based on the results of the described studies, vildagliptin was registered for use in patients with T2DM as monotherapy (in combination with diet therapy and exercise); in combination with metformin (as initial drug therapy); as part of a two-component combination therapy with metformin, PSM, thiazolidinediones or insulin, as well as as part of a triple combination therapy with metformin + PSM or metformin + insulin.
Vildagliptin
pharmachologic effect
Hypoglycemic agent. Vildagliptin is a representative of the class of stimulants of the islet apparatus of the pancreas, selectively inhibits the enzyme dipeptidyl peptidase-4 (DPP-4). Rapid and complete inhibition of DPP-4 activity (>90%) causes an increase in both basal and meal-stimulated secretion of glucagon-like peptide type 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) from the intestine into the systemic circulation throughout the day.
By increasing the levels of GLP-1 and GIP, vildagliptin causes an increase in the sensitivity of pancreatic β-cells to glucose, which leads to an improvement in glucose-dependent insulin secretion. When using vildagliptin at a dose of 50-100 mg/day in patients with type 2 diabetes mellitus, an improvement in the function of pancreatic β-cells is observed. The degree of improvement in β-cell function depends on the degree of initial damage; Thus, in individuals who do not have diabetes mellitus (with normal plasma glucose levels), vildagliptin does not stimulate insulin secretion and does not reduce glucose levels.
By increasing the levels of endogenous GLP-1, vildagliptin increases the sensitivity of α-cells to glucose, which leads to improved glucose-dependent regulation of glucagon secretion. Reducing the level of excess glucagon during meals, in turn, causes a decrease in insulin resistance.
An increase in the insulin/glucagon ratio against the background of hyperglycemia, caused by an increase in the levels of GLP-1 and GIP, causes a decrease in glucose production by the liver both during the prandial period and after a meal, which leads to a decrease in plasma glucose levels.
In addition, with the use of vildagliptin, a decrease in plasma lipid levels is observed, but this effect is not associated with its effect on GLP-1 or GIP and improvement of pancreatic β-cell function.
It is known that an increase in GLP-1 levels can lead to a delay in gastric emptying, but no such effect is observed with the use of vildagliptin.
When vildagliptin was used in 5795 patients with type 2 diabetes mellitus for 12 to 52 weeks as monotherapy or in combination with metformin, sulfonylureas, thiazolidinedione, or insulin, a significant long-term decrease in the concentration of glycated hemoglobin (HbA1c) and fasting blood glucose was observed.
Meta-analyses and comparative studies
The meta-analysis of the effectiveness of incretin therapy included 79 RCTs ( n
~40,000). The initial hba1c level was 7.2–9.3%, and ΔHbA1c was 0.6–1.1%. The effects of different SID-4s were similar, but vildagliptin showed the most pronounced reduction in HbA1c and HPG (Fig. 2)
Rice.
2. Average change in HbA1c (A) and FPG (B) levels while taking DPP-4 inhibitors [45]. [45]. To predict ΔHbA1c when selecting STD-4 based on a systematic review of 98 RCTs ( n
~24,000) a nomogram was developed. All other things being equal (hba1c and hpn), the highest ΔHbA1c was expected when choosing vildagliptin (Fig. 3)
Rice.
3. Nomogram for assessing ΔHbA1c when choosing DPP-4. How to use: (1) select an inhibitor and (2) initial HbA1c level, then (3) lower the perpendicular to the “points” scale, (4) similarly for FPG. (5) The sum of the baseline HbA1c and FPG scores corresponds to the expected reduction in HbA1c levels with the selected inhibitor [46]. [46]. The effectiveness of therapy is mainly assessed by the level of hba1c and hpn. However, mean glucose fluctuation (MAG) has an even greater impact on oxidative stress than hyperglycemia and is associated with the development of micro- and macrovascular complications [47, 48]. This was confirmed in two subanalyses of the DEVOTE study, which showed that SACH is associated with severe hypoglycemia as well as the risk of death from any cause [49].
In a 12-week study, patients with T2DM ( n
=90, hba1c >7.5%), receiving metformin at a dose of 2000 mg/day, were randomized into groups of intensification of therapy with sitagliptin 100 mg/day and vildagliptin 50 mg 2 times a day. Glycemic values were similar in both groups, but SACG was lower in the vildagliptin group [13]. The vildagliptin group had a greater reduction in nitrotyrosine levels and inflammatory markers, and multivariate analysis demonstrated a stronger correlation of these parameters with SACG than with postprandial glycemia. In addition, the increase in active GLP-1, β-cell response (auinsulin/aucglucose), and decrease in glucagon levels (prandial and interprandial) were more pronounced in the vildagliptin group than in the sitagliptin group (Fig. 4).
Rice.
4. Dynamics of GLP-1 and glucagon levels during the day [50]. [50]. In another study ( n
=73, metformin+psm therapy) the effect of adding vildagliptin and saxagliptin on SACG was studied.
With similar efficacy and safety of both drugs, vildagliptin reduced SACG more than saxagliptin. In the vildagliptin group, ΔSACG = 1.74 ± 0.48 mmol/l ( p
< 0.001), and in the saxagliptin group - 0.87 ± 0.40 mmol/l (
p
= 0.003).
Treatment with vildagliptin compared with sitagliptin and saxagliptin is associated with lower SACH. In addition, when taking vildagliptin, a greater decrease in glucagon levels was noted, and the patients' glycemia remained within the specified ranges longer [50, 51].
Maintaining glucose levels in the physiological range, according to the metabolic memory effect, helps reduce the risk of complications of T2DM and, possibly, reduce β-cell dysfunction.
The VERIFY trial is the first to evaluate the effect of early treatment with vildagliptin plus metformin on β-cell function. The durability of the effect is being studied in patients with newly or recently diagnosed T2DM who have a slight increase in hba1c levels (6.5-7.5%). The results of the study should show whether early dual therapy has long-term effects: increasing the time to treatment failure or to the initiation of insulin therapy, as well as an effect on vascular changes in patients with T2DM. Results are expected in 2021 [52].
Vildagliptin Medisorb
When using vildagliptin in monotherapy or in combination with other drugs, most adverse reactions were mild, temporary and did not require discontinuation of therapy. There was no correlation between the incidence of adverse reactions (HP) and age, gender, ethnicity, duration of use or dosage regimen.
The incidence of angioedema during vildagliptin therapy was ≥1/10,000.
The most common cases of angioedema were observed when using the drug in combination with angiotensin-converting enzyme inhibitors. In most cases, angioedema was of moderate severity and resolved spontaneously with continued therapy with vildagliptin.
Asymptomatic liver dysfunction (including hepatitis) has rarely been reported during vildagliptin therapy. In most cases, these disorders and deviations of liver function tests from normal resolved independently without complications after discontinuation of drug therapy. When using vildagliptin at a dose of 50 mg 1 or 2 times a day, the frequency of increases in the activity of liver enzymes (ALT or AST ≥3xULN) was 0.2% or 0.3%, respectively (compared to 0.2% in the control group). The increase in the activity of liver enzymes was in most cases asymptomatic, did not progress and was not accompanied by cholestasis or jaundice.
HP are grouped according to the MedDRA classification of organs and organ systems. Within each group of organs and organ systems, HPs are listed in order of decreasing frequency of occurrence. Within each group, the frequencies of HP are listed in order of decreasing severity.
To assess the incidence of HP, the following criteria were used: very often (≥1/10), often (≥1/100,
When using vildagliptin in monotherapy
When using vildagliptin at a dose of 100 mg per day, the frequency of discontinuation of therapy due to the development of adverse reactions (0.3%) was no higher than that in the placebo group (0.6%) or the comparator drug (0.5%).
During monotherapy with vildagliptin at a dose of 100 mg per day, the incidence of hypoglycemia without increasing the severity of the condition was 0.4%, which is comparable to the reference drug and placebo (0.2%).
Body weight did not change from baseline in clinical studies when vildagliptin 100 mg per day was used as monotherapy (-0.3 kg and -1.3 kg in the vildagliptin and placebo groups, respectively).
Infectious and parasitic diseases: very rarely - upper respiratory tract infections, nasopharyngitis.
Metabolic and nutritional disorders: infrequently - hypoglycemia.
Nervous system disorders: often - dizziness; infrequently - headache.
Gastrointestinal tract (GIT) disorders: uncommon - constipation.
Vascular disorders: uncommon - peripheral edema.
Musculoskeletal and connective tissue disorders: uncommon - arthralgia.
Long-term clinical studies of up to 2 years did not reveal any additional deviations in the safety profile or unexpected risks when using vildagliptin in monotherapy.
When using vildagliptin at a dose of 100 mg per day in combination with metformin
When using vildagliptin at a dose of 100 mg/day in combination with metformin or placebo in combination with metformin, there were no cases of discontinuation of therapy due to the development of adverse reactions.
When using vildagliptin at a dose of 100 mg per day in combination with metformin, hypoglycemia was observed in 1% of cases (in the placebo + metformin group, infrequently (0.4%)). No severe hypoglycemia was observed in the vildagliptin group.
Body weight did not change compared to baseline in clinical studies when using the combination of vildagliptin 100 mg per day and metformin (+0.2 kg and -1.0 kg in the vildagliptin and placebo groups, respectively).
Metabolic and nutritional disorders: often - hypoglycemia.
Nervous system disorders: often - tremor, headache, dizziness; infrequently - increased fatigue.
Gastrointestinal disorders: often - nausea.
Long-term clinical studies of up to 2 years did not reveal any additional safety profile deviations or unexpected risks when using vildagliptin in combination with metformin.
A study of the use of the combination of vildagliptin and metformin as initial therapy for type 2 diabetes did not reveal any abnormal safety profile or unexpected risks.
When using vildagliptin at a dose of 50 mg/day in combination with sulfonylurea derivatives
When using vildagliptin at a dose of 50 mg/day in combination with glimepiride, the rate of discontinuation of therapy due to the development of HP was 0.6% (compared to 0% in the glimepiride + placebo group).
The incidence of hypoglycemia in patients receiving vildagliptin 50 mg/day with glimepiride was 1.2% (compared to 0.6% in the placebo + glimepiride group). No severe hypoglycemia was observed in the vildagliptin group.
Body weight was unchanged from baseline in clinical studies when vildagliptin 50 mg once daily was added to glimepiride (-0.1 kg and -0.4 kg in the vildagliptin and placebo groups, respectively).
Infectious parasitic diseases: very rarely - nasopharyngitis.
Metabolic and nutritional disorders: often - hypoglycemia.
Gastrointestinal disorders: infrequently - constipation.
Nervous system disorders: often - tremor, dizziness, headache, asthenia.
When using vildagliptin at a dose of 100 mg per day in combination with a thiazolidinedione
When using vildagliptin at a dose of 100 mg/day + thiazolidinedione and placebo + thiazolidinedione, there were no cases of discontinuation of therapy due to the development of HP.
When using vildagliptin at a dose of 100 mg per day + pioglitazone, hypoglycemia was observed in 0.6% of cases, and in patients receiving placebo + pioglitazone - in 1.9% of cases. No severe hypoglycemia was observed in the vildagliptin group.
In a study of vildagliptin as add-on therapy to pioglitazone, the absolute weight gain in the placebo and vildagliptin 100 mg daily groups was 1.4 and 2.7 kg, respectively.
When vildagliptin 100 mg/day was added to pioglitazone 45 mg/day, the incidence of peripheral edema was 7% (compared to 2.5% with pioglitazone monotherapy).
Vascular disorders: often - peripheral edema.
Metabolic and nutritional disorders: often - weight gain; infrequently - hypoglycemia.
Nervous system disorders: uncommon - headache, asthenia.
When using vildagliptin at a dose of 50 mg 2 times a day in combination with insulin (with or without metformin)
When using the drug in combination with insulin (in combination with metformin or without metformin), the frequency of therapy discontinuation due to side effects was 0.3% in the vildagliptin therapy group; there were no cases of therapy discontinuation in the placebo group.
When using the drug in combination with insulin (in combination with metformin or without metformin), there was no increase in the risk of hypoglycemia compared with the combination of placebo + insulin (14% in the vildagliptin group and 16.4% in the placebo group). Two patients in the vildagliptin group and 6 patients in the placebo group developed severe hypoglycemia.
At the end of the study, the drug had no effect on average body weight (body weight increased by +0.6 kg compared to baseline in the vildagliptin group, and remained unchanged in the placebo group).
Nervous system disorders: often - headache, chills.
Gastrointestinal disorders: often - nausea, gastroesophageal reflux; infrequently - diarrhea, flatulence.
Metabolic and nutritional disorders: often - hypoglycemia.
When using vildagliptin at a dose of 50 mg twice a day in combination with sulfonylureas and metformin
There were no cases of drug withdrawal associated with HP in the combination therapy group with vildagliptin, metformin and glimepiride. In the placebo, metformin, and glimepiride combination therapy group, the HP-related discontinuation rate was 0.6%.
Hypoglycemia was common in both groups (5.1% in the vildagliptin, metformin and glimepiride combination group and 1.9% in the placebo, metformin and glimepiride combination group). There was one episode of severe hypoglycemia in the vildagliptin group.
At the end of the study, there was no significant effect on body weight (+0.6 kg in the vildagliptin group and -0.1 kg in the placebo group).
Nervous system disorders: often - dizziness, tremor.
Metabolic and nutritional disorders: often - hypoglycemia.
Disorders of the skin and subcutaneous tissues: often - hyperhidrosis.
General disorders and disorders at the injection site: often - asthenia.
Post-registration studies
During post-marketing studies, the following adverse reactions were identified (since the reports were obtained voluntarily from a population of unknown size, it is not possible to reliably determine the frequency of these HP developments, and therefore they are classified as 'frequency unknown').
Gastrointestinal disorders: frequency unknown - pancreatitis.
Disorders of the liver and biliary tract: frequency unknown - hepatitis (resolved independently after discontinuation of the drug), increased activity of liver enzymes (resolved independently after discontinuation of the drug).
Musculoskeletal and connective tissue disorders: frequency unknown - myalgia.
Skin and subcutaneous tissue disorders: frequency unknown - urticaria, exfoliative and bullous skin lesions, including bullous pemphigoid.
If you experience side effects listed in the instructions or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Safety. Use in special populations
Elderly patients
Elderly patients with T2DM are at high risk of micro- and macrovascular complications and cognitive impairment, and they require effective, safe and convenient therapy.
The INTERVAL study demonstrated the safety and effectiveness of STD-4 in patients over 70 years of age. The adjusted odds ratio of achieving the individual glycemic goal was 3.16 (1.81–5.52; p
<0.0001) in favor of vildagliptin, and the tolerability and effectiveness of glycemic control in elderly patients was similar to that in younger patients.
In a pooled database analysis of the use of vildagliptin in patients over 75 years of age, a reduction in HbA1c levels was demonstrated by 0.9% with monotherapy and by 1.1% with combination therapy with metformin. Among those receiving vildagliptin, AEs were less common than among those receiving the comparator drug [53].
Patients with chronic renal failure (CRF)
In patients with moderate to severe renal impairment, vildagliptin shows a good safety profile when taken during insulin therapy; in elderly patients (≥75 years); patients with newly diagnosed diabetes mellitus after transplantation; patients on hemodialysis [5].
In pharmacokinetic studies in patients with impaired renal function, similar plasma insulin concentrations were demonstrated with a 2-fold increased exposure to vildagliptin compared to patients with preserved renal function [4]. Since vildagliptin is excreted through the kidneys by 23% unchanged and 77% in the form of inactive metabolites and does not have a damaging effect on the kidneys in moderate (30 <GFR <50 ml/min) and severe (GFR <30 ml/min) chronic renal failure , the dose of vildagliptin can be reduced to 50 mg/day, while maintaining the effect and halving the cost of therapy.
In patients with T2DM with moderate and severe chronic renal failure, treatment with vildagliptin (50 mg/day) reduced HbA1c levels by 0.57 and 0.81%, respectively. A low risk of hypoglycemia was noted (26% in the moderate group and 18% in the severe chronic renal failure group). At the end of 52 weeks of vildagliptin therapy, no deterioration in renal function was observed, regardless of the initial degree of chronic renal failure [5].
Vildagliptin 50 mg/day was well tolerated in patients over 75 years of age ( n
=105) with moderate or severe chronic renal failure: ΔHbA1c was 1%, there was no increase in the frequency of hypoglycemia, and the frequency of other adverse events did not increase [54].
Patients with chronic heart failure (CHF)
The safety of vildagliptin in patients with CHF (NYHA functional class I-III) was studied in a 52-week double-blind RCT ( n
=254). It was found that treatment with vildagliptin does not affect left ventricular function and does not worsen the course of CHF. ΔHbA1c was 0.62% [55].
According to a network meta-analysis of the results of 50 RCTs [56], vildagliptin has the lowest chance of worsening heart failure (OR = 0.71).
Galvus Met tablets 50+850 mg 30 pcs. in Krasnoyarsk
Asymptomatic liver dysfunction (including hepatitis) has rarely been reported during vildagliptin therapy. In most cases, these disorders and deviations of liver function tests from normal resolved independently without complications after discontinuation of drug therapy. When using vildagliptin at a dose of 50 mg 1 or 2 times a day, the frequency of increases in liver enzyme activity (ALT or AST 3 times the ULN) was 0.2 or 0.3%, respectively (compared to 0.2% in the control group) . The increase in liver enzyme activity was in most cases asymptomatic, did not progress, and was not accompanied by cholestasis or jaundice.
To assess the incidence of adverse events (AEs), the following criteria were used: very common (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (<1/10000), including isolated cases.
Adverse reactions possibly associated with the use of combination therapy with vildagliptin and metformin (the incidence of which in the vildagliptin and metformin group differed from that in the placebo and metformin group by more than 2%) are presented below.
From the nervous system:
often - headache, dizziness, tremor.
When vildagliptin was used in combination with metformin in various doses, hypoglycemia was observed in 0.9% of cases (for comparison in the placebo group in combination with metformin - in 0.4%).
From the gastrointestinal tract frequency
AEs during combination therapy with vildagliptin and metformin were 12.9%. When using metformin, such AEs were observed in 18.1% of patients.
In the groups of patients receiving metformin in combination with vildagliptin, gastrointestinal disorders were observed with a frequency of 10–15%, and in the group of patients receiving metformin in combination with placebo, with a frequency of 18%.
Long-term clinical studies of up to 2 years did not reveal any additional safety profile abnormalities or unexpected risks when using vildagliptin as monotherapy.
A study of the use of a combination of vildagliptin and metformin as initial therapy for type 2 diabetes mellitus did not reveal risks and additional data on the safety of use.
Use of vildagliptin simultaneously with insulin
In controlled clinical trials using vildagliptin at a dose of 50 mg 2 times a day in combination with insulin in combination with or without metformin, the rate of discontinuation of therapy due to the development of adverse reactions was 0.3% in the vildagliptin group, while cases in the placebo group There was no discontinuation of therapy.
The incidence of hypoglycemia was comparable in both groups (14% in the vildagliptin group and 16.4% in the placebo group). Cases of severe hypoglycemia were observed in 2 patients in the vildagliptin group, and in 6 patients in the placebo group.
At the time of completion of the study, the drug did not have an effect on average body weight (body weight increased by 0.6 kg compared to baseline in the vildagliptin group, and no changes were noted in the placebo group).
Adverse events in patients receiving vildagliptin 50 mg twice daily in combination with insulin (with or without metformin) are presented below.
From the nervous system:
often - headache.
From the gastrointestinal tract: often
- nausea, gastroesophageal reflux; infrequently - diarrhea, flatulence.
Metabolism and nutrition:
often - hypoglycemia.
General and administration site disorders
: often - chills.
When using vildagliptin in combination with sulfonylureas
There were no cases of drug withdrawal associated with the development of AEs in the combination therapy group with vildagliptin, metformin and glimepiride. In the combination therapy group with placebo, metformin and glimepiride, the incidence of AEs was 0.6%.
Hypoglycemia was common in both groups (5.1% in the vildagliptin, metformin and glimepiride combination group and 1.9% in the placebo, metformin and glimepiride combination group). There was one episode of severe hypoglycemia in the vildagliptin group.
At the end of the study, there was no significant effect on body weight (+0.6 kg in the vildagliptin group and −0.1 kg in the placebo group).
AEs in patients receiving vildagliptin 50 mg 2 times a day in combination with metformin and sulfonylureas are presented below.
From the nervous system:
often - dizziness, tremor.
General disorders and disorders at the injection site:
often - fatigue.
Metabolism and nutrition:
often - hypoglycemia.
For the skin and subcutaneous tissues:
often - hyperhidrosis.
When using vildagliptin as monotherapy
From the nervous system:
often - dizziness; infrequently - headache.
From the gastrointestinal tract
: uncommon - constipation.
For the skin and subcutaneous tissues:
infrequently - skin rash.
From the musculoskeletal and connective tissue side
: often - arthralgia.
General disorders and disorders at the injection site:
infrequently - peripheral edema.
When using combination therapy with vildagliptin and metformin, there was no clinically significant increase in the frequency of the above-mentioned AEs observed when taking vildagliptin.
During monotherapy with vildagliptin or metformin, the incidence of hypoglycemia was 0.4% (uncommon).
Monotherapy with vildagliptin and combination treatment with vildagliptin + metformin had no effect on the body weight of patients.
Long-term clinical studies of up to 2 years did not reveal any additional safety profile abnormalities or unexpected risks when using vildagliptin as monotherapy.
Post-marketing studies
In the post-marketing period, the following adverse reactions were identified (since data are reported voluntarily from a population of unknown size, it is not possible to reliably determine the frequency of development of these AEs, and therefore they are classified as frequency unknown): hepatitis (reversible upon cessation of therapy), urticaria, pancreatitis, bullous and exfoliative skin lesions.
When using metformin in monotherapy
Metabolism and nutrition:
very often - loss of appetite; very rarely - lactic acidosis.
From the gastrointestinal tract:
very often - flatulence, nausea, vomiting, diarrhea, abdominal pain; often - dysgeusia.
From the liver and biliary tract:
very rarely - hepatitis.
For the skin and subcutaneous tissues:
very rarely - skin reactions (in particular erythema, itching, urticaria).
Laboratory and instrumental data
: very rarely - decreased absorption of vitamin B12, changes in liver function tests.
A decrease in the absorption of vitamin B12 and a decrease in its concentration in the blood serum during the use of metformin was observed very rarely in patients receiving the drug for a long time, and, as a rule, was not of clinical significance. The possibility of decreased absorption of vitamin B12 in patients with megaloblastic anemia should be considered.
Some cases of hepatitis that were observed during the use of metformin resolved after its discontinuation.
General security
Pooled analysis of data from 58 studies (vildagliptin, n
=10,331;
comparators, n
=8068) showed similar rates of AEs, serious AEs (SAEs), early treatment discontinuation, and deaths in both groups.
These data are supported by the results of the EDGE study ( n
= 45,868), in which the incidence of AEs was similar in the vildagliptin (5.3%) and comparator (5.7%) treatment groups [5].
Cardiovascular safety
The cardiovascular safety of vildagliptin was confirmed in a meta-analysis (40 phase III and IV studies, n
=17,000), which included patients with T2DM experience >10 years, patients over 65 years old, with impaired renal function and heart failure. It was shown that treatment with vildagliptin and comparators had similar risks in relation to the combined endpoint and its components (death from cardiovascular diseases, non-fatal myocardial infarction, non-fatal stroke) (Fig. 5)
Rice. 5. Mantel-Haenszel hazard ratios of the combined endpoint and its individual components during treatment with vildagliptin and comparator drugs [57]. [57].
Adverse skin effects
The pooled analysis did not reveal an increased incidence of cutaneous AEs with vildagliptin compared to other drugs. However, studies of pharmacovigilance databases indicate an increased incidence of bullous pemphigoid in older patients treated with IDPP-4, which is consistent with epidemiological observations that advanced age is a risk factor for the development of such lesions. When using STD-4 in patients with T2DM, monitoring of skin lesions is recommended [5].
Immune system safety
The CD26 receptor (with DPP-4 activity) is expressed on T lymphocytes and plays a role in the immune response. A similar frequency of infectious AEs and SAEs was noted with the use of vildagliptin and all comparator drugs. In a comprehensive review, vildagliptin did not increase the incidence of nasopharyngitis (OR=1.06 [0.93–1.21]), upper respiratory tract infections (OR=1.19 [0.98–1.45]) or urinary tract infections (OR=0.94 [0.57–1.56]) [5].
Pancreatic Safety
A meta-analysis of 69 studies showed no increased risk of pancreatitis during treatment with vildagliptin compared with comparators (OR=0.97 [0.37–2.53]). The risk of developing acute pancreatitis with STD-4 (except vildagliptin) compared with placebo was found in a meta-analysis (OR=1.79 [1.13-2.82]), although the absolute increase in risk was small (0.13%) [5].
Liver safety
The risk of a three-fold increase in ALT and AST levels, as well as the risk of developing AEs and SAEs from the liver during treatment with vildagliptin and comparator drugs were similar [5].
In the REDIRECT study in patients with T2DM (hba1c ≤7.6%), treatment with vildagliptin led to a decrease in hepatic triglyceride levels and a decrease in ALT. There was also a strong positive correlation ( r
=0.83;
p
< 0.0001) between a decrease in ALT levels and liver fat content [58].
Vildagliptin in real clinical practice (RCP)
The largest-scale study of the effects of IDPP-4 in the setting of RCP ( n
=45,000, 27 countries) is the EDGE study [59].
In the Russian part of the study ( n
= 1650), after 12 months of follow-up, 744 (81%) patients from the vildagliptin group and 460 (64%) from the comparison group achieved the primary endpoint.
While taking vildagliptin, the HbA1c level decreased by 1.3% (from 8.1 to 6.8%), in the comparison group - by 0.8% (from 8.0 to 7.2%) ( p
<0.0001 ). The frequency of hypoglycemia is 1.1 and 3.4, respectively. The majority of hypoglycemia in the vildagliptin group occurred during combination therapy with PSM. On vildagliptin therapy, more patients achieved target hba1c levels (<7%) without hypoglycemia or weight gain: 48.7% versus 19.1% [59].
A retrospective comparison of the results of therapy with metformin + vildagliptin and metformin + PSM was carried out. In both groups, the degree of decrease in hba1c correlated with its initial level. However, when taking PSM, the effectiveness demonstrated in the RCT (ΔHbA1c = 1.2%) was not fully reproduced in the RCP (ΔHbA1c 0.9%): the difference between the RCT and RCP was greater, the lower the initial hba1c level. At the same time, in patients in the vildagliptin group, ΔHbA1c (1.2% in RCT, 1.1% in RCP) was the same across the entire range of fluctuations in the initial hba1c level (Fig. 6).
Rice. 6. Efficacy of vildagliptin compared with PSM in addition to metformin - comparison of the results of RCTs and RCPs [53]. The discrepancy in the effectiveness of PSM in RCTs and RCPs could be due to low patient adherence to treatment and slow dose titration, possibly due to fear of hypoglycemia or weight gain.
The GUARD study assessed the efficacy, safety, and tolerability of vildagliptin alone or in combination with metformin in PCP ( n
=19,000). A decrease in hba1c levels occurred at all stages of T2DM (Fig. 7)
Rice.
7. Average ΔHbA1c according to the GUARD study; *p<0.0001 compared to baseline [60]. regardless of gender, age and degree of obesity of patients [60]. Another study (PROVIL) compared the effectiveness and safety of the combination of vildagliptin + metformin and other glucose-lowering therapy in the setting of RC. Patients ( n
=3648, duration of T2DM ~6 years, hba1c level 7.6-7.9%) were distributed into three treatment groups: 1st - vildagliptin + metformin (
n
=603);
2nd fixed combination of vildagliptin + metformin in one tablet ( n
= 2198) and 3rd - other PSSPs (PSM + metformin, thiazolidinediones + metformin, other IDPP-4 + metformin) (
n
= 847). After 6 months, a statistically significant decrease in HbA1c levels was observed in all groups, but with vildagliptin therapy the effect was more pronounced (ΔHbA1c=0.9%). In patients receiving vildagliptin, there was a decrease in body weight by 1.4 and 1.7 kg (by 0.8 kg in the comparison group); the number of AEs and hypoglycemia was comparable in all groups. The results of the study confirmed the effectiveness of combination therapy with vildagliptin and metformin in the setting of RCP without an increase in the number of AEs [61].
A retrospective cohort study involving more than 20 thousand patients with T2DM showed that vildagliptin therapy compared with PSM was associated with a significantly lower risk of developing retino- and neuropathies, and there was also a trend towards a decrease in nephropathies and ulcerations associated with diabetic foot (Fig. . 
Rice. 8. Risk ratio of T2DM complications when taking vildagliptin and PSM [62]. [62].
In an analysis of data from 52,750 patients with T2DM (Swedish drug, cause of death and patient registries), the combination of metformin+PSM was associated with a greater risk of severe hypoglycemia, cardiovascular events and death from any cause than the combination of metformin+PSM. -4 [63].
A retrospective analytical study of 5 European databases assessed the cardiovascular safety of vildagliptin compared with other NSAIDs. The analysis included data from more than 730 thousand patients with T2DM. The results showed no increase in the risk of myocardial infarction, acute coronary syndrome, stroke, or CHF when taking vildagliptin compared with other PSAPs [64].
Dipeptidyl peptidase-4-vildagliptin inhibitor - a new drug in the treatment of type 2 diabetes mellitus
In many patients with type 2 diabetes mellitus (T2DM), the use of oral hypoglycemic drugs (ODGs) does not achieve the necessary glycemic control. According to the UKPDS study, disease compensation 3 years after diagnosis with PSSP monotherapy was achieved in only 45% of patients, and after 6 years - in only 30%. This circumstance dictates the need to develop and introduce new drugs that not only eliminate metabolic disorders, but also maintain the functional activity of pancreatic cells, stimulating and activating the physiological mechanisms of insulin secretion and blood glucose control.
The regulation of glucose homeostasis in the body is carried out by a complex polyhormonal system, including pancreatic hormones and incretin hormones produced in the intestines in response to food intake. Up to 70% of postprandial insulin secretion in healthy people is due precisely to the effect of incretins () [1]. In patients with type 2 diabetes, this effect is significantly reduced ().
The incretins that play a major role in the insulin response to food are glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1).
The entry of food into the digestive tract quickly stimulates the release of GIP and GLP-1, which, due to their joint action:
- stimulate the release of insulin by beta cells and inhibit the production of glucagon by alpha cells of the pancreas in response to increased blood glucose;
- increase the concentration of insulin, promoting the utilization of glucose by peripheral tissues;
- reduce the release of glucose by the liver.
Incretins can also reduce glycemic levels through “non-insulin” mechanisms by slowing gastric emptying and reducing food intake.
In type 2 diabetes, the content of incretins and their effect are reduced. However, the effect of incretins in such patients can be restored by administering exogenous drugs [3]. Therefore, the result of elucidating the role of gastrointestinal hormones (incretins) in the regulation of carbohydrate metabolism was the creation of a fundamentally new class of glucose-lowering drugs - incretin mimetics (Fig. 3).
New recommendations for the selection of treatment regimens and algorithms for maintaining glycemic control, presented at the International Diabetes Federation Congress (Montreal, October 18–23, 2009), emphasized the importance of dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 agonists. Drugs from these groups are recommended at almost all stages of treatment for type 2 diabetes.
One of the representatives of the class of DPP-4 inhibitors is the drug vildagliptin (Galvus®), registered in Russia in November 2008. Vildagliptin is used in patients with type 2 diabetes as monotherapy at a dose of 50 mg or 100 mg per day or in combination with metformin , sulfonylureas (SMU) or insulin.
Selective and reversible inhibition of the DPP-4 enzyme increases the concentration of endogenous GIP and GLP-1, and also prolongs the period of their presence in plasma. Vildagliptin thus enhances the physiological effects of both incretin hormones, improving glucose-dependent insulin secretion and reducing increased glucagon secretion. Reducing excess glucagon levels during meals, in turn, causes a decrease in insulin resistance.
Inhibition of DPP-4 has been shown after a single dose of vildagliptin in healthy volunteers and multiple doses in patients with type 2 diabetes. In this case, the duration of inhibition was dose-dependent. Doses of 50 mg and 100 mg once daily were equally effective in inhibiting DPP-4 during the daytime meal, but the 100 mg dose was more effective during the night period.
Increases in GLP-1 and GIP levels have been demonstrated in patients with type 2 diabetes treated with vildagliptin for 4 weeks; There was an increase in incretin levels after meals and on an empty stomach. Elevated levels were observed both during the day and at night.
The main clinical effect of the drug is an improvement in glycemic control, a decrease in the level of glycated hemoglobin HbA1c (an integral indicator of the control of carbohydrate metabolism for 2–3 months). This allows more patients to achieve therapeutic treatment goals. The effects of lowering glycemic levels and HbA1c have been studied using various methods (fasting and postprandial plasma glucose levels, 24-hour glycemic profile, endogenous glucose production). Vildagliptin reduced postprandial plasma glucose levels in patients with type 2 diabetes, both in monotherapy and in combination with sulfonylureas or metformin, the most commonly used drugs [1]. In addition, while taking vildagliptin (4 weeks), patients with type 2 diabetes experienced a decrease in serum triglyceride levels.
Thus, we can talk about the multicomponent action of vildagliptin, which includes:
- DPP-4 inhibition (main effect);
- increased levels of GLP-1 and GIP (desired effect);
- effects on pancreatic islet function (increased insulin response of beta cells and decreased glucagon response of alpha cells to glucose) and insulin resistance;
- influence on the glycemic profile (decrease in fasting and postprandial glucose levels) and endogenous glucose production;
- decreased lipid levels after meals;
- influence on gastric emptying.
More than 20 thousand patients took part in clinical trials of vildagliptin, with 14 thousand patients directly receiving the drug. All studies were controlled, multicenter, double-blind, parallel group.
Vildagliptin versus rosiglitazone as monotherapy
The effectiveness and tolerability of vildagliptin and rosiglitazone therapy were compared over 24 weeks of therapy [8]. Patients included in the study, who had not previously received drug therapy, with a baseline HbA1c of 7.5–11.0% were randomized to therapy with vildagliptin (50 mg twice a day) or rosiglitazone (8 mg once a day). The study included 697 patients with type 2 diabetes (vildagliptin n = 459; rosiglitazone n = 238). The main parameter of the study was the dynamics of HbA1c: at baseline and after 24 weeks. Secondary study parameters: dynamics of fasting plasma glucose, fasting plasma lipid levels, dynamics of body weight, incidence of edema during therapy.
The results of the study showed a significant decrease in HbA1c levels compared to baseline values by -1.1% and -1.3% for vildagliptin and rosiglitazone, respectively (p < 0.001 for both drugs) ().
In patients with initially high HbA1c (> 9%), a significantly greater decrease was shown in both treatment groups: -1.8% in the vildagliptin therapy group (initial 10.0%) and -1.9% in the rosiglitazone therapy group (initial 9.9%). At the same time, there were statistically significant (p < 0.001) differences in changes in body weight between treatment groups: vildagliptin did not affect body weight, taking rosiglitazone led to its increase by an average of 4.7 kg. A higher incidence of peripheral edema was found in the rosiglitazone group (11.1%) compared to the vildagliptin group (4.6%). The results showed that with equal glucose-lowering effectiveness, vildagliptin provides better tolerability.
Addition of vildagliptin to metformin therapy compared with PSM or PSM + metformin therapy
The effectiveness and safety of vildagliptin (50 mg ´ 2 times / day) and glimepiride (a drug from the sulfonylurea group, used at a dose of up to 6 mg / day) was studied with long-term use (52 weeks) in patients with poor glycemic control (HbA1c 6.5 –8.5%) when taking metformin (constant dose ~1.9 g/day). A year later, there was a decrease in HbA1c in both groups (vildagliptin -0.4%; glimepiride -0.5%) () [9]. Thus, when added to metformin, vildagliptin showed similar efficacy to glimepiride.
In addition, the body weight of patients taking vildagliptin remained stable over the course of the study, whereas weight gain was observed in patients taking glimepiride. The difference between groups at 52 weeks was 1.8 kg. After 52 weeks of therapy, no weight gain was observed in patients receiving vildagliptin + metformin.
Particular attention was paid to comparing the number of hypoglycaemias in both groups: 1.7% of patients in the vildagliptin group (n = 1389) and 16.2% of patients in the glimepiride group (n = 1383) experienced ≥ 1 hypoglycemic episode ().
There were 39 hypoglycemic episodes reported in the vildagliptin group and 554 in the glimepiride group. No severe (grade 2 or close to grade 2) hypoglycemic episodes were observed in the vildagliptin group, whereas 10 severe hypoglycemic episodes were recorded in the glimepiride group. At the end of 52 weeks, fewer hypoglycemic episodes were observed in the vildagliptin group [10].
Vildagliptin + NPH insulin
A 24-week study compared the effects of vildagliptin (50 mg twice daily, n = 144) or placebo (n = 152) in patients with type 2 diabetes receiving insulin therapy (for at least 3 months at a dose > 30 U/day) and did not achieve adequate glycemic control (initial HbA1c 7.5–11%). The main parameter of this study was to evaluate the effectiveness of adding vildagliptin to insulin in terms of the effect on reducing HbA1c in comparison with insulin monotherapy ().
Overall, the addition of vildagliptin to insulin significantly reduced HbA1c levels compared with baseline values and with the monotherapy group. The between-group difference was -0.3% (P = 0.01). Patients in the subgroup over ≥ 65 years of age also showed a significant and more pronounced decrease in glycemia than in the vildagliptin + insulin group: -0.7% HbA1c compared to baseline values (p < 0.001).
In addition, it was proven that significantly fewer patients in the vildagliptin + insulin therapy group had episodes of hypoglycemia (23% of patients reported 113 episodes; P < 0.01) compared with placebo + insulin therapy (30% of patients 185 episodes reported). In the vildagliptin + insulin therapy group there was not a single episode of severe hypoglycemia compared to the insulin + placebo therapy, where 6 episodes were noted (). Thus, despite the pronounced hypoglycemic effect, vildagliptin reduces the frequency of hypoglycemic reactions, especially severe ones.
Vildagliptin + metformin
The combination of vildagliptin + metformin achieved a reduction in HbA1c levels by 1.1% compared to the group taking metformin + placebo [2, 3]. Patients who do not have adequate glycemic control on metformin monotherapy are 4 times more likely to achieve disease compensation with the combined use of metformin and vildagliptin compared to the combination of metformin + placebo [4]. In addition, the addition of vildagliptin to metformin therapy has been shown to be as effective as the use of metformin with thiazolidinediones (TZDs). At the same time, no weight gain was observed [5] ().
The effectiveness of long-term use of vildagliptin was confirmed by a study of mono- and combination therapy lasting 52 weeks ().
The results obtained with the combined use of vildagliptin and metformin substantiated the idea of creating the combined drug GalvusMet® (vildagliptin + metformin), which was registered in Russia in March 2009. GalvusMet® is prescribed in cases where monotherapy with metformin does not produce a significant result. Vildagliptin in combination with metformin provides reliable control of blood glucose levels. GalvusMet® is available in three dosages: vildagliptin 50 mg + metformin at a dose of 500 mg, 850 mg or 1000 mg. Taken 2 times a day.
Frequency of development of undesirable side effects during vildagliptin therapy
The overall incidence of adverse events was comparable in the vildagliptin + metformin and placebo + metformin groups. A decrease in the incidence of gastrointestinal (GIT) disorders was observed with the combination of vildagliptin + metformin in 15% of cases compared to the combination of placebo + metformin - 18%. The incidence of adverse events did not increase with the duration of therapy. The incidence of serious adverse events was low and comparable for both groups. Of the clinically significant adverse events, headache was more common when taking vildagliptin than when taking placebo + metformin. There was no worsening of the side effects of metformin when vildagliptin was added to therapy.
In combination therapy studies, vildagliptin was not associated with an increased incidence of hypoglycemia when combined with metformin. The percentage of patients with hypoglycemic events in the vildagliptin + glimepiride group was slightly higher than in the corresponding placebo group (2.4% versus 0.6%). The observed hypoglycemia was of mild severity and did not lead to discontinuation of the drug. As expected, a significantly higher incidence of hypoglycemia was observed in the insulin study, but the incidence was lower in the vildagliptin + insulin group (23%) than in the placebo + insulin group (30%). In the “placebo + insulin” group, a greater number of hypoglycemic reactions were of moderate severity. Several severe episodes were noted in this same group.
Compared with metformin, patients with pre-existing cardiovascular risk factors (cardiovascular disease, hypertension and/or dyslipidemia) did not show a higher incidence of death, myocardial infarction (MI) or stroke when taking vildagliptin. Certain cardiovascular disorders (hypertension, significant ECG abnormalities) did not increase with mono- or combination therapy.
Thus, the main properties of vildagliptin in relation to unwanted side effects are:
- tolerability and safety similar to placebo, without increasing the overall incidence of adverse events in relation to major systems and organs, both in mono- and combination therapy;
- better tolerability from the gastrointestinal tract compared to metformin;
- no increase in the risk of hypoglycemia: the drug did not increase the frequency of hypoglycemia in monotherapy and in combination with insulin, demonstrated a smaller number and milder severity of hypoglycemia episodes than insulin + placebo, did not show an increase in the frequency of hypoglycemia episodes in combination with metformin.
In addition, vildagliptin does not increase cardiovascular risk, does not cause weight gain, increased incidence of edema or heart failure, has a neutral effect on fasting lipids, and has a positive effect on postprandial lipid profiles.
Conclusion
The current epidemiological situation is characterized, on the one hand, by a significant increase in the incidence of type 2 diabetes, and on the other hand, by the insufficient effectiveness of existing treatment methods. In this situation, the development and introduction of new drugs for the treatment of type 2 diabetes are becoming increasingly important. The role of innovative drugs with original mechanisms of action will be increasingly important, as they open up new opportunities for correcting pancreatic islet dysfunction in type 2 diabetes and influencing the progression of the disease. Vildagliptin, being a representative of a new class of drugs for the treatment of type 2 diabetes, has a number of advantages compared to traditional therapy. By suppressing the activity of DPP-4, the drug increases the level of incretin hormones (GLP-1 and GIP). In turn, their increased levels, by increasing insulin levels and decreasing glucagon levels, allow for more effective blood glucose control and a decrease in glucose production by the liver. This leads to the most important clinical effect - achieving disease compensation (decrease in HbA1c).
Literature
- Garber A. et al. Effects of Vildagliptin on Glucose Control in Patients with Type 2 Diabetes Inadequately Controlled with a Sulphonylurea // Diabetes, Obesity and Metabolism. 2008; 10: 1326–1463.
- Bosi E. et al. Effects of Vildagliptin on Glucose Control Over 24 Weeks in Patients with Type 2 Diabetes Inadequately Controlled with Metformin // Diabetes Care. 2007; 30:890–895.
- Stratton I. et al. Association of Glycaemia with Macrovascular and Microvascular Complications of Type 2 Diabetes (UKPDS 35): Prospective Observational Study // BMJ. 2000; 321:405–412.
- Dejager S. et al. Achievement of Glycemic Targets with Vildagliptin // Presented at EASD, 17–21 September 2007 (Abstract A-07–899).
- Bolli G. et al. Efficacy and Tolerability of Vildagliptin vs. Pioglitazone when Added to Metformin: A 24-Week, Randomized, Double-Blind Study // Diabetes, Obesity and Metabolism. 2008; 10: 82–90.
- Garber A. et al. Efficacy and Tolerability of Vildagliptin Added to a Sulfonylurea (SU) in Patients with Type 2 Diabetes (T2 DM) // Presented at ADA, June 22–26, 2007 (Abstract 501-P).
- Rosenstock J. et al. Efficacy and Tolerability of Initial Combination Therapy with Vildagliptin and Pioglitazone Compared with Component Monotherapy in Patients with Type 2 Diabetes // Diabetes, Obesity and Metabolism. 2007; 9 (2): 175–185.
- Rosenstock J., Baron MA, Dejager S. et al. Comparison of vildagliptin and rosiglitazone Monotherapy in patients with Type 2 Diabetes: a 24-week, double-blind, randomized trial // Diabetes Care. 2007, 30: 217–223.
- Ferrannini E. et al. 52-week efficacy and safety of vildagliptin versus glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009 (in press).
- Fonseca V. et al. Addition of vildagliptin to insulin improves glycemic control in type 2 diabetes // Diabetologia. 2007; 50: 1148–1155.
M. B. Antsiferov , Doctor of Medical Sciences, Professor L. G. Dorofeeva , Doctor of Medical Sciences
Endocrinological dispensary of the Department of Health, Moscow
Contact information for authors for correspondence
Additional Information
Source of financing.
Search and analytical work and preparation of the article were carried out by the author at his own expense.
Conflict of interest.
Demidova T.Yu. - lecturer, participant in expert councils, clinical studies of Astra Zeneca, Novo Nordisk, Sanofi, Novartis, Boehringer Ingelheim, Takeda, Berlin Chemi.
Information about authors
Demidova Tatyana Yulievna
- Doctor of Medical Sciences, Prof. [Tatiana Yu. Demidova, MD, PhD, Professor]; Address: Russia, 117997, Moscow, st. Ostrovitianova, 1, [address: 1 Ostrovitianova street, 117997 Moscow, Russia]; telephones; SCOPUS: 7003771623; SPIN code: 9600-9796; e-mail