Chemical properties
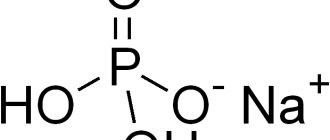
Sodium Citrate, what is it? To understand what sodium salt of citric acid is, we need to consider its structure. Chemical formula of the substance: Na3C6H5O7. The product has the appearance of a white fine-crystalline powder, has a salty-sour taste, and is odorless. Melts at 310 degrees Celsius. Molecular weight = 258 grams per mole. Tri-substituted Sodium Citrate is highly soluble in water, soluble in alcohols, and insoluble in organic solutions.
Harm and benefit
The chemical compound is actively used:
- as a seasoning, spice, preservative under code E331 , in the production of lemon-flavored soda, energy drinks, gelatin desserts, in baby food;
- as a buffer to maintain a stable pH, as an anticoagulant, in coffee machines;
- in pharmaceuticals, it is part of soluble forms of drugs, for example, for heartburn ;
- as a laxative in the treatment of genitourinary infections;
- in analytical chemistry, when determining ESR;
- in windshield wiper fluids.
Sodium Citrate, as a rule, does not cause harm to the human body. The substance is non-toxic and does not cause irritation upon contact with skin. It is better not to inhale the product. There have been no recorded cases of poisoning with this chemical compound.
Impact on the body
In general, foods with high acidity can cause internal inflammation, while alkali acts in the opposite direction - suppressing inflammatory processes. Therefore, it is very important to take into account the pH level of each product in order to take care of your own health.
Under normal conditions, our blood has an alkaline pH. To maintain an optimal balance, you should build your daily diet so that the proportion of alkaline foods is 75%.
| Proportion of alkaline foods in the daily diet | Proportion of acidic foods in the daily diet |
| 75% | 25% |
Sodium citrate, entering the body, reacts with lymph, blood alkali and bile. Provided the acid-base balance is maintained, the body copes with the processing of substances. But if the norm of acidic components is significantly exceeded, not all of them can be neutralized with alkali. In such a situation, a disruption of metabolic processes in the body occurs, which can be accompanied by such unpleasant symptoms as swelling of the mucous membrane of the upper respiratory tract, headaches, neuralgic disorders, loss of appetite
Speaking about the issue of the benefits and harms of sodium citrate, it should be remembered that negative consequences arise only in the case of a significant excess of consumption of the substance in comparison with alkaline components.
special instructions
Treatment courses with Sodium Citrate should not be repeated frequently. If symptoms persist after therapy, it is recommended to change treatment tactics.
It is not recommended to prescribe the medicine to men and children, due to the fact that the disease is often bacterial in nature.
Particular caution is recommended when treating patients with cardiovascular diseases, high blood pressure, kidney disease, diabetes mellitus , and following a salt-free diet.
Dessert recipe using sodium citrate - “Chocolate Spheres” (caviar)
"Chocolate spheres" (caviar)
Ingredients:
- sodium citrate - 0.5 g;
- sugar - 90 g;
- water - 200 g;
- sodium alginate - 3 g;
- calcium lactate - 3 g;
- cocoa powder - 50 g.
Tools:
bowls, pipette, blender
Preparation:
1. Using a blender, mix sodium citrate and sodium alginate in water. 2. Add cocoa powder and sugar. Stir on high speed until smooth. 3. Remove any air bubbles by gently tapping the bowl on the table. 4. Separately prepare a calcium bath. 5. Using a pipette, scoop up the cocoa mixture, drop it into the calcium bath, and leave the caviar for 30 seconds. 6. Using a slotted spoon, remove and rinse in water.
Goes very well with ice cream.
Drugs containing (Analogs)
Level 4 ATC code matches: Sodium bicarbonate
Trade names of the product: Blemaren , disubstituted Sodium Citrate , Sodium Citrate dihydrate . The substance is contained in the following drugs: Trihydron , Glyugitsir , Gidrovit Forte , Regidron , Faglucid , CFDA-1 , Kafanol , Ionica , Microlax , Re-Sol , Electral .
Production of sodium citrate
Modern methods of producing sodium citrate depend on the required amount of the finished substance. For small production volumes, sodium citrate is created through citric acid fermentation, which is subjected to waste generated during sugar production.
Sugar and sodium citrate production are often placed side by side, since molasses and molasses after sugar production are used to produce sodium citrate.
Production in large volumes is high-tech and requires high energy and water consumption. The main route of industrial production is biosynthesis from sugar or sugary substances (molasses) by industrial strains of the mold Aspergillus niger.
Previously, the additive was produced from shag biomass and lemon juice.
Receiving[ | ]
In industry[ | ]
Since the mid-1800s. citric acid was obtained exclusively from the juice of unripe lemons, mixing it with quicklime and thus precipitating poorly soluble calcium citrate. Treatment of calcium citrate with sulfuric acid leads to the formation of a calcium sulfate precipitate, and citric acid was isolated from the supernatant by crystallization. The yield of this process was 2-3 wt. % of the dry weight of fruits[6]. The literature mentions that citric acid in the form of calcium salt was transported from Sicily and Southern Italy to places of consumption (mainly England, France and the USA), and the acid itself was isolated on the spot [7].
In 1893, the first enzymatic method for producing citric acid was discovered: the German chemist and mycologist Karl Wemer used molds of the Penicillium genus for this. However, it was not possible to introduce the method into industry due to problems with purifying the product. Success was achieved only in 1919, when the enzymatic process was organized in Belgium. The preponderance in favor of enzymatic production occurred after the First World War, when problems arose with the supply of citric acid from Italy, and global needs were increasingly increasing. In 1923, Pfizer commercialized the process of converting carbohydrates into citric acid under the influence of mold fungi of the species Aspergillus niger,
in the presence of a small amount of inorganic salts[7].
As of the beginning of the 21st century. the entire volume of industrial citric acid is produced by biosynthesis. The raw materials used are corn hydrolysate (in North and South America and Europe), cassava, sweet potato and corn hydrolysate (in Asia), crystalline sucrose (in South America) and molasses (in Asia and Europe). In some cases, citric acid is obtained from agricultural waste[6].
This process has been used since the 1930s. Theoretically, from 100 kg of sucrose you can get 123 kg of citric acid monohydrate or 112 kg of anhydrous citric acid. In fact, the yield is lower, since the fungi consume some of the sucrose for their own growth and respiration. The actual yield ranges from 60 to 85% of the theoretical one. The enzymatic process can be carried out in three types:
- solid state fermentation;
- surface fermentation;
- deep fermentation[8].
In solid state fermentation
the raw materials are placed in troughs and moistened with water. If necessary, nutrients are added to the water, and then a fungal culture is placed there. After the process is completed, the citric acid is washed out with water, separated from the solution and purified.
Surface fermentation
carried out on special trays where the substrate and some inorganic nutrients are placed. The media is adjusted in the range of 3-7 pH depending on the type of substrate, then sterilization is carried out and the required temperature is set. Then a culture of fungi is applied to the trays, which multiplies and covers the entire surface of the substrate, after which the formation of citric acid begins. At the end of the process, citric acid is separated from the liquid.
Deep fermentation
carried out in large containers in two stages. First, 10% of the substrate is fermented for 1 day as a seed, after which the mixture is added to the bulk and fermented for 3-7 days. The process is carried out by constantly blowing the liquid with air using a compressor[8].
After fermentation, the liquid is filtered through a membrane and the citric acid is separated from proteins and residual carbohydrates using quicklime, extraction or chromatography. According to the first, most common method, citric acid is precipitated in the form of a calcium salt, which is then treated with sulfuric acid, producing insoluble gypsum and a solution of purified citric acid. The second method is based on the use of a specific solvent in which citric acid dissolves better than impurities.
Chromatographic purification is based on the use of anion exchangers: citric acid is sorbed on a carrier and then washed out of the sorbent with dilute sulfuric acid [9].
After isolation, purification is carried out. To do this, contaminated citric acid is treated with activated carbon to remove colored impurities, passed through a layer of ion exchange resins to remove soluble salts, filtered from insoluble impurities and crystallized [8].
In 2012, global citric acid production was approximately 1.6 million tons, of which approximately 0.8–0.9 million tons were produced in China. About 70% of total production is used in the food industry[8].
Laboratory synthesis[ | ]
In the classic laboratory synthesis of citric acid, acetone is used as a starting material, which is brominated at methyl groups, then reacted with hydrogen cyanide and hydrolyzed[6].
Total synthesis[ | ]
Citric acid was first produced by chemical synthesis by Grimaux and Adam in 1880. The starting compound in this synthesis was glycerol. The primary hydroxyl groups of the glycerol molecule were first replaced by chlorine atoms, and then by nitrile groups, which upon hydrolysis gave terminal carboxyl groups. The secondary hydroxyl group was oxidized to a keto group, to which hydrogen cyanide was then added; the resulting cyanohydrin also yielded a carboxyl group upon hydrolysis[10].
Another approach was proposed in 1890. It was based on the conversion of acetoacetic ester, which was chlorinated, as expected, at the terminal α-position, then a nitrile group was introduced into the same position, which was eventually hydrolyzed to a carboxyl group. At the last stage, substituents were created at the C2 atom, obtaining cyanohydrin and hydrolyzing it in an acidic environment. The scheme of this total synthesis was questioned: some chemists, for example, Charles Prevost, suggested that it was not the terminal α-position of the acetoacetic ester that was chlorinated, but the middle one, which resulted in the formation of not citric acid itself, but its isomer. The debate is believed to have arisen due to the fact that at the end of the 19th century. There were no spectroscopic methods yet that would allow us to notice this difference [11].
In 1891, citric acid was obtained by adding hydrocyanic acid to acetone dicarboxylic acid monoethyl ester followed by hydrolysis. True, the original substance itself was originally obtained from citric acid [12].
In 1897, an approach to the synthesis of citric acid was proposed, based on the recently discovered reaction of Reformatsky (1895). According to this method, ethyl bromoacetate and diethyl oxaloacetate were introduced into the reaction in the presence of zinc [13].
Of the more recent approaches, we can note the transformation of oxaloacetic acid proposed in 1973, which upon self-condensation with decarboxylation gave citroylformic acid. The latter is then in the presence of hydrogen peroxide or tert
-butyl hydroperoxide was converted into citric acid[14][15].
In 1980, citric acid was obtained by the condensation reaction of 3-methylbuten-3-ol-1 and formaldehyde, followed by oxidation of the resulting product with nitrogen dioxide[16][15].
Being in nature[ | ]
Citric acid is found in various fruits, in large quantities in citrus fruits (about 5% in fruits and up to 9% in juice). 100 g of lime contains 7 g of citric acid; lemon - 5.6 g; raspberries - 2.5 g; black currant - 1.2 g; tomatoes - 1.0 g; pineapple and strawberries - 0.6 g; cranberries - 0.2 g; apple - 14 mg[2].
Citric acid is involved in the tricarboxylic acid cycle, the main process of cell respiration, so it is found in some noticeable concentration in the body of all animals and plants [2]. The tricarboxylic acid cycle or the citric acid cycle or the Krebs cycle is the main chemical mechanism for producing a universal source of ATP in animal and human mitochondria.
Application
Sodium citrate E331 is widely used in the food industry. In the production of processed cheese as a melting salt in an amount of up to 30 g/kg of processed cheese. Citrates give processed cheese a pleasant, slightly sour taste and a moderately dense, elastic consistency. A decrease in pH creates unfavorable conditions for the life of gas-forming microorganisms, as a result of which processed cheese using citrates is more stable during storage.
As a stabilizer for minced meat products up to 3 g/kg of meat or fat. To restore the salt (ionic) balance necessary for the thermal stability of milk subjected to heating, stabilizer salts are added to it, which can be all citrates that bind calcium ions. Salts are used in the form of 10-25% aqueous solutions. Tri-substituted sodium citrate most effectively restores salt balance. The dose of stabilizer salt depends on the heat resistance of a particular batch of milk, therefore it ranges from 0.05-04% of the mass of the mixture being normalized.
Sodium citrate E331 is also used as an acidity regulator and retarder in marmalade, jams, preserves, pectin jellies, desserts, bakery, confectionery products, etc. up to 10 g/kg; antioxidant synergist in juices, margarines, vegetable oils, lard, pork fat 100 mg/kg. Acceleration of the dissolution of dry mixtures (for example, for ice cream) is achieved by adding phosphates or sodium citrates.
Sodium citrate
trisubstituted is included in the list of raw materials for margarine; canned milk; cocoa with condensed milk and sugar; sterilized condensed milk; whole condensed milk.
Notes[ | ]
- Apelblat, 2014, p. 1.
- ↑ 123
NULLmann, 2014, p. 1. - ↑ 12
NULLmann, 2014, p. 2. - ↑ 12
NULLmann, 2014, p. 3. - CRC Handbook of Chemistry and Physics / W. M. Haynes, editor-in-chief. — CRC Press, 2016-2017. — P. 5-90.
- ↑ 12345
NULLmann, 2014, p. 4. - ↑ 12
Apelblat, 2014, p. 2. - ↑ 1234
NULLmann, 2014, p. 4–6. - Ullmann, 2014, p. 6–7.
- Apelblat, 2014, p. 213.
- Apelblat, 2014, p. 214–215.
- Apelblat, 2014, p. 216.
- Apelblat, 2014, p. 215.
- Wiley RH, Kim KS
Bimolecular decarboxylative self-condensation of oxaloacetic acid to citrolyformic acid and its conversion by oxidative decarboxylation to citric acid: [English] // J. Org. Chem.. - 1973. - Vol. 38, no. 20. - P. 3582–3585. - doi:10.1021/jo00960a030. - ↑ 12
Apelblat, 2014, p. 216–217. - Wilkes JB, Wall RG
Reaction of dinitrogen tetraoxide with hydrophilic olefins: synthesis of citric and 2-hydroxy-2-methylbutanedioic acids: [English] // J. Org. Chem.. - 1980. - Vol. 45, no. 2. - P. 247–250. - doi:10.1021/jo01290a008. - Singh, N. B.;
AK Singh, S. Prabha Singh. Effect of citric acid on the hydration of portland cement (English) // Cement and Concrete Research : journal. - 1986. - Vol. 16, no. 6. - P. 911-920. - ISSN 00088846. - doi:10.1016/0008-8846(86)90015-3. - Kozlova V.K., Karpova Yu.V., Wolf A.V.
Evaluation of the effectiveness of additives that retard the setting of cement paste // Polzunovsky Bulletin. - 2006. - Issue. No. 2—2. — P. 230-233. - RadioKot :: Safe, publicly available composition for etching copper at home
- https://www.jstor.org/pss/2749354
- Ullmann, 2014, p. 8.
- Merck Safety Data Sheet - Citric acid (pdf)
- Record of Zitronensäure
in the GESTIS Substance Database of the IFA, accessed on 10. January 2021.
How can I replace E331?
Manufacturers of canned fish and meat replace sodium citrate with triphosphates (E451) or orthophosphoric acid (E452). The first substance regulates acidity and acts as an emulsifier, the second increases the shelf life of products.
Pyrophosphates (E450) are added to minced meat and canned food so that they remain fresh longer.
E339 is often added to milk, butter, and bread to preserve the properties of the product. An analogue of E331 is sodium carbonate, which is considered a preservative for meat products.
Additive E451.
Main manufacturers
The domestic market for food sodium citrates is 50% represented by products from Chinese manufacturers. Among them, Anhui Fengyuan Biochemical Co. is the leader. Ltd, which has developed its own highly effective technologies for producing the additive.
More than 1000 tons of E 331 additive per year are produced by the Russian enterprise Citrobel (Belgorod).
A large dose of ammonium acetate can be fatal.
If you see a food additive under the symbol E151 in your favorite product, then you should refuse this delicacy. Why - read here.
Without olives you cannot prepare many delicious dishes. How to choose them correctly, read this article.
Type of substance
Due to its technological functions, food additive E 331 is included in the group of antioxidants.
It is usually used as an antioxidant synergist, emulsifier, and pH regulator. Sodium citrates are a group of substances that differ from each other in chemical structure, molecular weight, and acidity level.
The following types are distinguished:
- 1-substituted sodium citrate or monosodium citrate (aqueous and anhydrous), E331(i), formula NaC6H6O7;
- 2-substituted sodium citrate, disodium citrate, (aqueous), E331(ii), formula Na2C6H6O7∙1.5∙H2O;
- 3-substituted sodium citrate, trisodium citrate (aqueous and anhydrous), E331(iii), formula Na3C6H6O7.
The food industry typically uses forms E331(ii) and E331(iii). They have a long shelf life, a high content of the main substance, and a small amount of impurities.
Monosodium citrate is used for medical needs.
The raw material for the production of food additive E 331 is citric acid. It is neutralized to a slightly alkaline reaction with soda ash or sodium hydroxide.
How to identify an additive in a product?
Since sodium citrate is not a prohibited food additive, you can find out about its presence in products by looking at the labeling on the label. Manufacturers, as a rule, do not hide the presence of this food additive in the product.
Most often denoted as:
- sodium citrate;
- Monosodium Citrate;
- Sodium hydrogen citrate;
- sodium hydrogen citrate;
- Trisodium Citrate;
- Disodium Citrate;
- Sodium Citrates;
- sodium citrate;
- E-331, E331.
Interesting Facts
There are several interesting facts associated with the dietary supplement:
- It is resistant to high temperatures, therefore it is used for the manufacture of dairy products that require long-term heat treatment.
- Constant consumption of foods with sour salt leads to disruption of the taste receptors. People consider artificial food more appetizing than natural food.
- The substance is used in alpine skiing. Skiers receive it before the descent to avoid muscle acidification.
Acid salt is used in molecular gastronomy. It is necessary for chefs to create sphere-shaped desserts, as it helps maintain the unusual shape.