Home / Food additives (E) / Antioxidants (E-300 - E-399)
Back
Published: 10/02/2016
Reading time: 9 min
0
2214
Supplement E 383 combines two vital elements: calcium and phosphorus. It would seem that an antioxidant should be present in many products. For a long time it was like that. But then the food industry was banned from using the synthetic substance.
Let's look at the reasons for this decision.
- The product's name
- Type of substance
- Properties
- Package
- Application
- Benefits and harms
- Main manufacturers
Type of substance
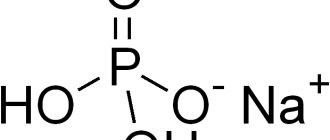
Calcium glycerophosphate is included in the category of antioxidants.
Until 2010, in food production it performed the technological function of a thickener and consistency stabilizer. Food additive E 383 belongs to the pharmacological group of micro- and macroelements.
The substance is obtained through a multi-stage chemical process. At the initial stage, glycerol is esterified with phosphoric acid. The resulting ester reacts with calcium chloride or other soluble salts of the macroelement. Final crystallization and purification yields the pharmaceutical raw material, calcium glycerophosphate.
Arava tablets 10 mg 30 pcs ➤ instructions for use
Arava should only be prescribed to patients after a thorough medical examination. Before starting treatment with Arava, you must be aware of the possible increase in the number of side effects in patients who have previously received other basic drugs for the treatment of rheumatoid arthritis, which have hepato- and hematotoxic effects. The active metabolite of leflunomide, A771726, has a long half-life, usually ranging from 1 to 4 weeks. Due to the long half-life of leflunomide's active metabolite, A771726, serious adverse effects (e.g., hepatotoxicity, hematoxicity, or allergic reactions, see below) may occur or persist even if leflunomide treatment is discontinued. In this case, a “washing” procedure should be carried out. The procedure can be repeated according to clinical indications. If severe immunological/allergic reactions such as Stevens-Johnson syndrome or Lyell's syndrome are suspected, a complete “washing” procedure is mandatory. Therefore, if such cases of toxicity occur or when switching to another basic drug (for example, methotrexate) after treatment with leflunomide, it is necessary to carry out a washout procedure (see below).
Liver reactions Because the active metabolite of leflunomide, A771726, is protein bound and eliminated through hepatic metabolism and bile secretion, it is expected that plasma levels of A771726 may be increased in patients with hypoproteinemia. Arava is contraindicated in patients with severe hypoproteinemia or impaired liver function. (see section "Contraindications".). Rare cases of severe liver damage, in some cases fatal, have been reported during treatment with leflunomide. Most of these cases were observed during the first six months of treatment. Although the causal relationship of these adverse events to leflunomide has not been established, and in most cases there were several additional suspicious factors, strict adherence to treatment monitoring recommendations is considered mandatory. ALT levels should be checked before starting leflunomide therapy and then every 2 weeks for the first 6 months of treatment, followed by once every 6 to 8 weeks. There are the following recommendations for adjusting the dosage regimen or discontinuing the drug, depending on the severity and persistence of the increase in ALT levels. If ALT is confirmed to be 2-3 times the upper limit of normal, reducing the dose from 20 mg to 10 mg per day may allow leflunomide to be continued, provided that this indicator is carefully monitored. If 2-3 times the upper limit of normal ALT persists, or if there is a confirmed rise in ALT levels greater than 3 times the upper limit of normal, leflunomide should be discontinued and a washout procedure should be initiated. Due to possible additive hepatotoxic effects, it is recommended to avoid alcohol intake during treatment with leflunomide.
Hematological reactions A complete clinical blood count, including determination of the leukocyte formula and platelet count, should be performed before starting treatment with leflunomide, as well as every 2 weeks during the first 6 months of treatment and then every 6-8 weeks. In patients with pre-existing anemia, leukopenia and/or thrombocytopenia, as well as in patients with impaired bone marrow function or at risk of developing such disorders, the risk of hematological disorders increases. If this type of phenomenon occurs, a “washing” procedure should be used to reduce the level of A771726 in the blood plasma. If serious hematological reactions, including pancytopenia, occur, discontinue Arava and any other concomitant drug that suppresses bone marrow hematopoiesis and initiate a washout procedure.
Concomitant use with other treatments There is currently no information available regarding the concomitant use of leflunomide with antimalarial drugs used in rheumatology (for example, chloroquine and hydroxychloroquine), intramuscular or oral gold preparations, D-penicillamine, azathioprine and other immunosuppressive drugs (except methotrexate). The risk associated with the prescription of complex therapy, especially with long-term treatment, is unknown. Since this type of therapy can lead to the development of additive or even synergistic toxicity (for example, hepato- or hematotoxicity), combinations of this drug with other basic drugs (for example, methotrexate) are not advisable.
Switching to other treatments Because leflunomide remains in the body for a long time, switching to another disease-modifying drug (eg, methotrexate) without an appropriate washout may increase the possibility of additional risks even long after switching (eg, kinetic interactions, organ toxicity). Likewise, recent treatment with hepatotoxic or haematoxic drugs (eg methotrexate) may result in an increased incidence of adverse events, so when starting treatment with leflunomide, it is necessary to carefully consider all the positive and negative aspects associated with taking this drug.
Skin reactions If ulcerative stomatitis develops, leflunomide should be discontinued. Very rare cases of Stevens-Johnson syndrome or toxic epidermal necrolysis have been reported in patients receiving leflunomide. If skin and/or mucosal reactions occur, you should stop taking Arava and any other related drug and immediately begin a washout procedure. It is necessary to achieve complete elimination of the drug from the body. In such cases, re-prescribing the drug is contraindicated.
Infections It is known that drugs like leflunomide, which have immunosuppressive properties, make patients more susceptible to various types of infections, including opportunistic infections (infections caused by fungi and microorganisms that can cause infections only in conditions of decreased immunity). Infectious diseases that arise are usually severe and require early and intensive treatment. If a severe infection occurs, it may be necessary to interrupt leflunomide treatment and initiate a washout procedure. Tuberculin-positive patients should be closely monitored due to the risk of reactivation of tuberculosis.
Respiratory tract reactions Rare cases of interstitial pulmonary disease have been reported during leflunomide therapy. Symptoms such as cough and dyspnea may be a reason to stop taking leflunomide.
Blood pressure Blood pressure levels should be monitored before starting treatment with leflunomide and periodically after starting it.
Interactions Caution should be exercised when prescribing drugs metabolized by CYP2C9 (phenytoin, warfarin, tolbutamide), with the exception of NSAIDs (non-steroidal anti-inflammatory drugs).
Recommendations for men There is no data on the risk of fetotoxicity (associated with the toxic effect of the drug on the father's sperm) when using leflunomide in men. Experimental data in this direction have not been carried out. To minimize the possible risk, men planning to have a child should stop taking leflunomide and use cholestyramine 8 g 3 times a day for 11 days or 50 g of powdered activated carbon 4 times a day for 11 days.
Package
Calcium glycerophosphate powder with a volume of 5 to 25 kg is packaged in cardboard winding drums or aluminum barrels. A polyethylene bag is inserted inside. The container must be hermetically sealed.
A nutritional supplement weighing up to 1 kg is packaged in plastic or foil bags and brewed to prevent moisture from entering.
Store in a cool, dry place.
Calcium glycerophosphate is available for retail sale in the form of tablets or granules for self-preparation of a suspension.
Properties
| Index | Standard values |
| Color | white |
| Compound | calcium (about 19%), phosphorus, impurities (heavy metals in small doses); empirical formula C3H7Ca06P |
| Appearance | fine crystalline powder |
| Smell | absent |
| Solubility | good in cold water, especially in the presence of acids (diluted hydrochloric, citric, lactic); Almost insoluble in ethanol, ethers, boiling water, chloroform |
| Main substance content | 99% |
| Taste | bitter |
| Density | not determined |
| Other | becomes cloudy when heated; decomposes when boiled |
Benefits and harms
Antioxidant E 383 is useful as a mineral supplement, a source of phosphorus and calcium. The nutrient stimulates cellular renewal, strengthens bones and the nervous system, and increases the body's resistance to adverse factors.
The special value of the supplement is that calcium glycerophosphate is absorbed into the walls of the gastrointestinal tract in a free (ionized) form, which is physiologically active.
The substance can only be taken after consultation with a doctor.
Calcium, phosphorus and glycerin are considered allergens.
Uncontrolled use of the food additive E 383 can provoke the development of diseases dangerous to health:
- hypercalcemia;
- thrombosis and thrombophlebitis;
- violation of the acidity level of gastric juice;
- diarrhea;
- joint inflammation;
- renal failure.
Dietary supplement E304 is a fat-soluble form of vitamin C.
When consumed in moderation, calcium acetate may benefit your health. Read more about this here.
Nicotinic acid has nothing to do with a dangerous alkaloid. You will find detailed information about this dietary supplement in this article.
Main manufacturers
Calcium glycerophosphate as a medicinal and vitamin preparation is produced by many domestic enterprises (Veropharm, Kaustik, Uralbiopharm and others). All of them use foreign raw materials.
As an intermediate chemical ingredient, food additive E 383 is supplied by:
- IBIS CHEMIE INTERNATIONAL company (India, Maharashtra);
- industrial group INDO GULF COMPANY (India, states of Maharashtra and Gujar);
- Shaanxi Yuantai Biological Technology Co. (China);
- Xi'an Kerui Biotechnology Co. (China).
Calcium glycerophosphate is a synthetic substance. Like any product of modern chemistry, it can be equally beneficial and harmful.
It is safer to replenish calcium reserves in the body by daily consumption of its natural sources: cottage cheese, milk, nuts, cereals.
Application
Until 2010, antioxidant E 383 was used in food production as a thickener and emulsifier. More often it was added to various fats, sauces, and confectionery products.
Amendments made to SanPiN 2.3.2.2795-10 number 168 dated December 23, 2010 prohibited the use of calcium glycerophosphate in the food industry due to possible side effects and pronounced anabolic effects.
Antioxidant E 383 was approved as a specialized additive in sports nutrition by the Decree of the Chief Sanitary Doctor of the Russian Federation dated January 27, 2010 . It stimulates the synthesis of amino acids in the body, increases endurance, and promotes the growth of muscle tissue.
The main areas of application of calcium glycerophosphate are:
- medicine. The additive is used in the complex treatment of increased fatigue, nervous disorders, malnutrition, and hypocalcemia. Prescribed to children over two years of age for dystrophy, neurasthenia and other ailments.
- veterinary medicine (treatment and prevention of rickets);
- cosmetic industry (as one of the components of toothpastes that strengthen tooth enamel).
The acceptable daily intake has not been determined.
There is no information on tolerances in the Codex Alimentarius.
Glycerophosphates
Details Category:
GLYCEROPHOSPHATES
, salts of glycerinophosphoric acid. Glycerol phosphoric acid C3H9O6P is a glycerol ester of phosphoric acid and forms two isomers:
α-acid is optically active, obtained from the hydrolysis of lecithins, and is found in free form in small quantities in normal urine; β-acid is optically inactive, is obtained synthetically and is not identical in properties to α-acid. Glycerinophosphoric acid is widely distributed in the plant and animal kingdoms: in combination with choline C5H15NO2 and high molecular weight fatty acids, it forms lecithins - substances found in egg yolk, nervous tissue, blood cells, etc.
Glycerophosphates are used in medicine as tonics and agents that strengthen the nervous system: in the body they break down into glycerol and phosphoric acid, but can also remain undecomposed and serve as material for the construction of lecithins and nucleins, etc. arr. they participate in assimilation and increase metabolism. Glycerophosphates are used either in pure form or in various mixtures, for example, Bauer's sanatogen - a mixture of 50% sodium glycerophosphate with casein. Glycerophosphates are soluble in water and insoluble in alcohol. Their solutions are precipitated with lead acetate. When boiled with mineral acids, glycerophosphates break down into glycerol and phosphoric acid. When heated to 130° they are stable, but when heated they turn into pyrophosphates.
Calcium glycerophosphate C3H7O6RSa·H2O is a white powder consisting of a mixture of α- and β-isomers. It is less soluble in hot water than in cold water. The solubility of commercial salt is highly variable depending on the relative content of α- and β-isomers, of which the β-isomer is the least soluble. To obtain calcium glycerophosphate, equivalent amounts of glycerin and 66% phosphoric acid are heated in a vacuum at 105°. The resulting syrupy mass is treated with lime milk and filtered from the precipitated phosphate. The filtrate is evaporated and precipitated with alcohol.
Sodium glycerophosphate C3H7O6PNa2·7H2O is obtained by heating 130 parts of sodium phosphate NaH2PO4·H2O with 230 parts of glycerol in an iron vacuum apparatus to 145° and then boiling the reaction product with a solution of sodium hydroxide. Excess glycerin is removed by steam distillation. Upon cooling, β-salt crystals precipitate. A syrupy α-salt remains in the mother liquor. Sodium glycerophosphate is sold either in the form of white crystals of the β-salt or in the form of a 50-70% solution of the α-salt.
In addition to calcium and sodium glycerophosphates, glycerophosphates of iron, lithium and magnesium are used in medicine.
Source: Martens. Technical encyclopedia. Volume 5 - 1929
- < Back
- Forward >