Nise gel (1% 50g)
INSTRUCTIONS for use of the medicinal product for medical use NIZE®
Registration number: P N012824/02
Trade name: Nise®
International nonproprietary name: nimesulide
Chemical name: N-(4-nitro-2-phenoxyphenyl) methanesulfonanilide
Dosage form: gel for external use
Composition: 1 g of gel contains: active substance: nimesulide 10 mg; excipients: N-methyl-2-pyrrolidone 250 mg, propylene glycol 100 mg, macrogol 315.5 mg, isopropanol 100 mg, purified water 200 mg, carbomer-940 20 mg, butylated hydroxyanisole 0.2 mg, thiomersal 0.1 mg, potassium dihydrogen phosphate 0.2 mg, flavor (Narcissus-938) 4 mg.
Description: transparent gel of light yellow or yellow color, free from foreign particles.
Pharmacotherapeutic group: non-steroidal anti-inflammatory drug (NSAID)
ATX code: M01AX17
Pharmacological action Nise® gel is a new generation non-steroidal anti-inflammatory drug (NSAID) from the sulfonamide class. It has a local analgesic and anti-inflammatory effect. Nimesulide is a selective competitive reversible inhibitor of cyclooxygenase type II (endoperoxide-prostaglandin-H2 synthetase). Reduces the concentration of short-lived prostaglandin H2, a substrate for kinin-stimulated synthesis of prostaglandin E2, at the site of inflammation and in the ascending pathways of pain impulses in the spinal cord. A decrease in the concentration of prostaglandin E2 (a mediator of inflammation and pain) reduces the activation of EP type prostanoid receptors, which is manifested by analgesic and anti-inflammatory effects. When applied topically, it causes a weakening or disappearance of pain at the site of application of the gel, including pain in the joints at rest and during movement, reduces morning stiffness and swelling of the joints. Helps increase range of motion.
Pharmacokinetics When applying the gel, the concentration of the active substance in the systemic circulation is extremely low. The maximum concentration after a single application is observed at the end of the first day; its value is more than 300 times lower than that for oral dosage forms of nimesulide. No traces of the main metabolite of nimesulide, 4-hydroxynimesulide, are found in the blood.
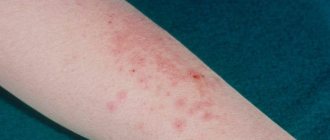
Indications Local symptomatic treatment of inflammatory and degenerative diseases of the musculoskeletal system (articular syndrome with exacerbation of gout, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, osteoarthritis, osteochondrosis with radicular syndrome, radiculitis, inflammatory damage to ligaments, tendons, bursitis, sciatica, lumbago). Muscle pain of rheumatic and non-rheumatic origin. Post-traumatic inflammation of soft tissues and the musculoskeletal system (damage and rupture of ligaments, bruises).
Contraindications Hypersensitivity to nimesulide and the components of the drug; erosive and ulcerative lesions of the gastrointestinal tract in the acute stage, bleeding from the gastrointestinal tract, dermatoses, damage to the epidermis and skin infections in the area of application; severe renal (creatinine clearance less than 30 ml/min.) or liver failure, a history of bronchospasm associated with the use of acetylsalicylic acid or other NSAIDs, pregnancy and lactation, children under 7 years of age.
With caution : Liver failure; renal failure; severe heart failure; arterial hypertension; diabetes mellitus type 2; elderly and children's age.
Directions for use and dosage : Externally. Before applying the gel, wash and dry the skin surface. Apply a uniform thin layer of gel approximately 3 cm long to the area of maximum pain, without rubbing, 3-4 times a day. The amount of gel and the frequency of its use (no more than 4 times a day) may vary depending on the size of the treated area and the patient’s response. Do not use the gel for more than 10 days without consulting a doctor.
Side effects Local reactions: itching, urticaria, peeling, transient change in skin color (not requiring discontinuation of the drug). If any adverse reactions occur, you should stop using the drug and consult your doctor. When applying the gel to large areas of the skin or with prolonged use, the development of systemic adverse reactions is possible: heartburn, nausea, vomiting, diarrhea, gastralgia, ulceration of the gastrointestinal mucosa, increased activity of “liver” transaminases; headache, dizziness; fluid retention, hematuria; allergic reactions (anaphylactic shock, skin rash); thrombocytopenia, leukopenia, anemia, agranulocytosis, prolongation of bleeding time.
Overdose Cases of drug overdose have not been described. However, when large quantities of gel (exceeding 50 g) are applied to large areas of skin, the development of an overdose cannot be ruled out. There is no specific antidote. You need to see a doctor.
Interaction with other drugs Pharmacokinetic interaction with drugs that compete for association with blood plasma proteins is not excluded. Caution should be exercised when using Nise® simultaneously with digoxin, phenytoin, lithium preparations, diuretics, cyclosporine, methotrexate, other NSAIDs, antihypertensive and antidiabetic agents. Before using the gel, you should consult your doctor if you are using these products or are under medical supervision.
Special instructions The drug is recommended to be applied only to intact areas of the skin, avoiding contact with open wounds. Avoid getting the gel into the eyes and other mucous membranes. Do not use the gel under airtight dressings. After applying the gel, wash your hands with soap. Close the tube tightly after using the gel.
Release form Gel for external use 1%. 20 g or 50 g in a laminated aluminum tube equipped with a membrane to control the first opening. The tube is packed in a cardboard box with instructions for use.
Shelf life: 2 years. Do not use the drug after the expiration date indicated on the package.
Storage conditions List B. In a dry place, protected from light, at a temperature not exceeding 25 °C. Do not freeze. Keep out of the reach of children!
Conditions for dispensing from pharmacies Without a prescription.
Manufacturer Dr. Reddy's Laboratories Ltd., Hyderabad, Andhra Pradesh, India
Manufacturing address Dr. Reddy's Laboratories Ltd. Plot No. 41, Nasigere Village, Kasaba Hobli, KIADB, Malur-563130, Karnataka, India.
Send consumer complaints to the following address: Representative office: 115035, Moscow, Ovchinnikovskaya embankment, 20, building 1 tel., 783-29-01 fax
Nise
NSAIDs. A selective inhibitor of COX-2, an enzyme involved in the synthesis of prostaglandins - mediators of edema, inflammation and pain. The drug has anti-inflammatory, analgesic and antipyretic effects.
Reversibly inhibits the formation of prostaglandin E2, both at the site of inflammation and in the ascending pathways of the nociceptive system, including the pathways of pain impulses in the spinal cord.
Reduces the concentration of short-lived prostaglandin H2, from which prostaglandin E2 is formed under the action of prostaglandin isomerase. A decrease in the concentration of prostaglandin E2 leads to a decrease in the degree of activation of EP-type prostanoid receptors, which is expressed in analgesic and anti-inflammatory effects.
It has a slight effect on COX-1 and practically does not interfere with the formation of prostaglandin E2 from arachidonic acid under physiological conditions, thereby reducing the number of side effects of the drug.
The drug suppresses platelet aggregation by inhibiting the synthesis of endoperoxides and thromboxane A2, and inhibits the synthesis of platelet aggregation factor. Suppresses the release of histamine and also reduces the degree of bronchospasm caused by exposure to histamine and acetaldehyde.
The drug also inhibits the release of tumor necrosis factor alpha, which causes the formation of cytokines.
It has been shown that nimesulide is able to suppress the synthesis of interleukin-6 and urokinase, thereby preventing the destruction of cartilage tissue. Inhibits the synthesis of metalloproteases (elastase, collagenase), preventing the destruction of proteoglycans and collagen of cartilage tissue.
It has antioxidant properties and inhibits the formation of toxic oxygen breakdown products by reducing the activity of myeloperoxidase. Interacts with glucocorticoid receptors, activating them through phosphorylation, which also enhances the anti-inflammatory effect of the drug.
When applied topically, it causes a weakening or disappearance of pain at the site of application of the gel, incl. pain in the joints at rest and during movement, reduces morning stiffness and swelling of the joints. Helps increase range of motion.
Pharmacokinetics
Suction
After oral administration, nimesulide is well absorbed from the gastrointestinal tract. Eating reduces the rate of absorption without affecting its extent. Cmax of nimesulide in blood plasma is achieved 1.5-2.5 hours after administration and is 3.5-6.5 mg/l. Subject to a first-pass effect through the liver.
Distribution
Binding to plasma proteins is 95%, to erythrocytes - 2%, to lipoproteins - 1%, to acidic α1-glycoproteins - 1%. The dose of the drug does not affect the degree of binding to blood proteins.
Vd is 0.19-0.35 l/kg. Penetrates into the tissues of the female genital organs, where after a single dose the concentration of nimesulide is about 40% of the concentration in plasma. Penetrates well into the acidic environment of the inflammation site (40%) and synovial fluid (43%). Easily penetrates histo-hematological barriers.
Metabolism
Nimesulide is actively metabolized in the liver by tissue monooxygenases. The main metabolite, 4-hydroxynimesulide (25%), has similar pharmacological activity.
Removal
T1/2 - 1.56-4.95 hours, T1/2 of 4-hydroxynimesulide - 2.89-4.78 hours. The metabolite is excreted by the kidneys (65%) and bile (35%).
Pharmacokinetics in special clinical situations
In patients with renal failure (creatinine clearance from 80 to 30 ml/min), as well as in children and the elderly, the pharmacokinetic profile of nimesulide does not change significantly.